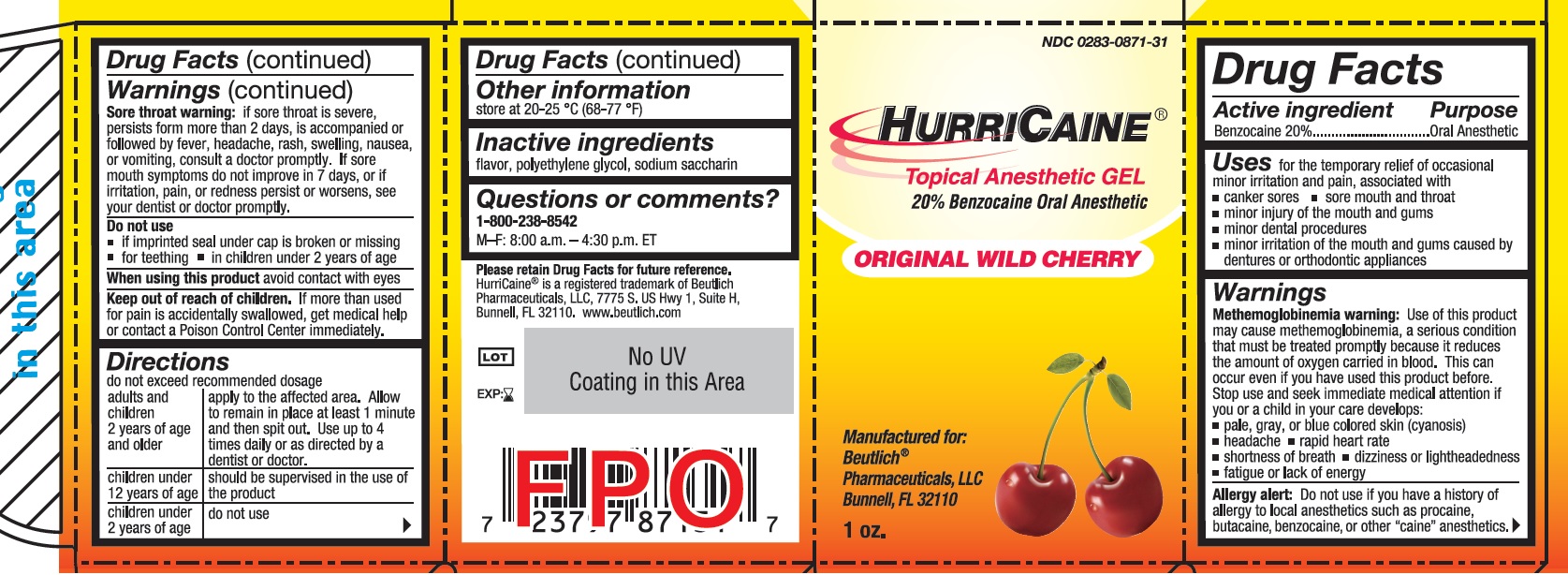
Uses
for the temporary relief of occasional minor irritation and pain, associated with
- canker sores
- sore mouth and throat
- minor injury of the mouth and gums
- minor dental procedures
- minor irritation of the mouth and gums caused by dentures or orthodontic appliances
Warnings
Allergy alert: Do not use if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Stop use and ask a doctor
- if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting
- if sore mouth symptoms do not improve in 7 days, or irritation, pain, or redness persists or worsens
Keep out of reach of children.
If more than used for pain is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- do not exceed recommended dosage
adults and children 2 years of age and older: apply to the affected area. Allow to remain in place at least 1 minute and then spit out. Use up to 4 times daily or as directed by a dentist or doctor.
children under 12 years of age: should be supervised in the use of the product
children under 2 years of age: consult a dentist or doctor