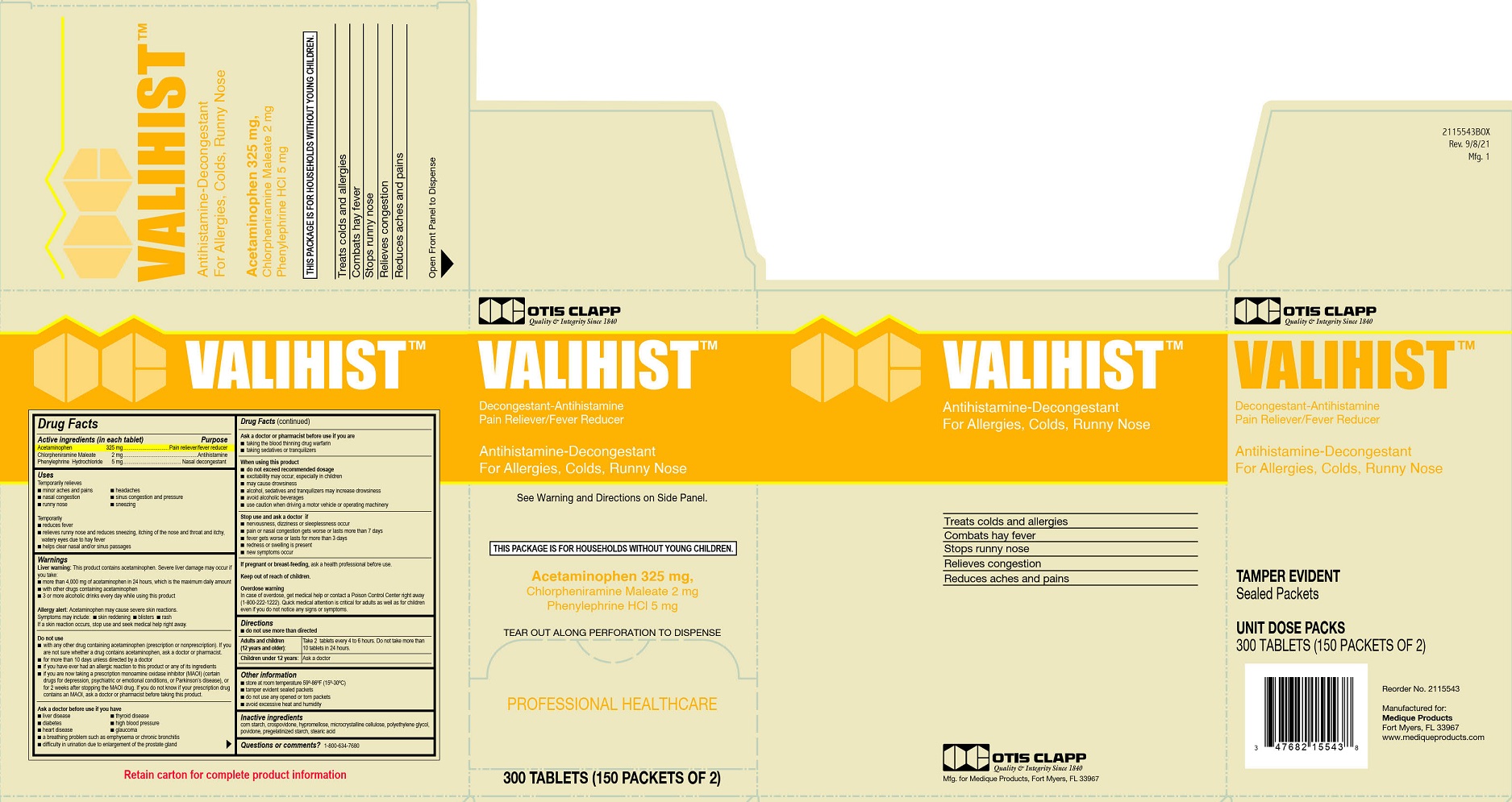
Active ingredients (in each tablet)
Acetaminophen 325 mg
Chlorpheniramine Maleate 2 mg
Phenylephrine Hydrochloride 5 mg
Uses
Temporarily relieves
- minor aches and pains
- headache
- nasal congestion
- sinus congestion and pressure
- runny nose
- sneezing
Temporarily
- reduces fever
- relieves runny nose and reduces sneezing, itching of the nose and throat and itchy, watery eyes due to hay fever
- helps clear nasal and/or sinus passages
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- for more than 10 days for pain unless directed by a doctor
- if you ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
liver disease
thyroid disease
diabetes
high blood pressure
heart disease
glaucoma
chronic bronchitis or emphysema
difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- may cause drowsiness
- alcohol, sedatives and tranquilizers may increase drowsiness
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- nervousness, dizziness or sleeplessness occur
- pain or nasal congestion gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
Keep out of reach of children.
Overdose warning
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-12220. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- do not use more than directed
Adults and children (12 years and older): Take 2 tablets every 4 to 6 hours. Do not take more than 10 tablets in 24 hours.
Children under 12 years: Ask a doctor
Other information
- store at room temperature 59º-86ºF (15º-30ºC)
- tamper evident sealed packets
- do not use any opened or torn packets
- avoid excessive heat and humidity
Inactive ingredients
corn starch, crospovidone, hypromellose, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, stearic acid
Principal Display Panel - Otis Clapp Valihist Label
Otis Clapp
Quality and Integrity Since 1840
VALIHIST ™
Decongestant-Antihistamine
Pain Reliever-Fever Reducer
Antihistamine- Decongestant
For Allergies, Colds, Runny Nose
See Warnings and Directions on Side Panel
This Package is for Households without Young Children.
Acetaminophen 325 mg,
Chlorpheniramine Maleate 2mg
Phenylephrine HCl 5 mg
Tear Out Along Perforation To Dispense
PROFESSIONAL HEALTHCARE
300 TABLETS (150 PACKETS OF 2)
Principal Display Panel - Medique Medicidin D Label
Medique®
Medicidin-D
Cold and Allergy Relief
This Package is for Households without Young Children.
Pull to Open
Pain Reliever/ Fever Reducer ● Acetaminophen 325mg
Antihistamine ● Chlorpheniramine Maleate 2mg
Nasal Decongestant ● Phenylephrine HCl 5mg
100Tablets
(50 x 2)
Tamper Evident Unit Dose Packets
