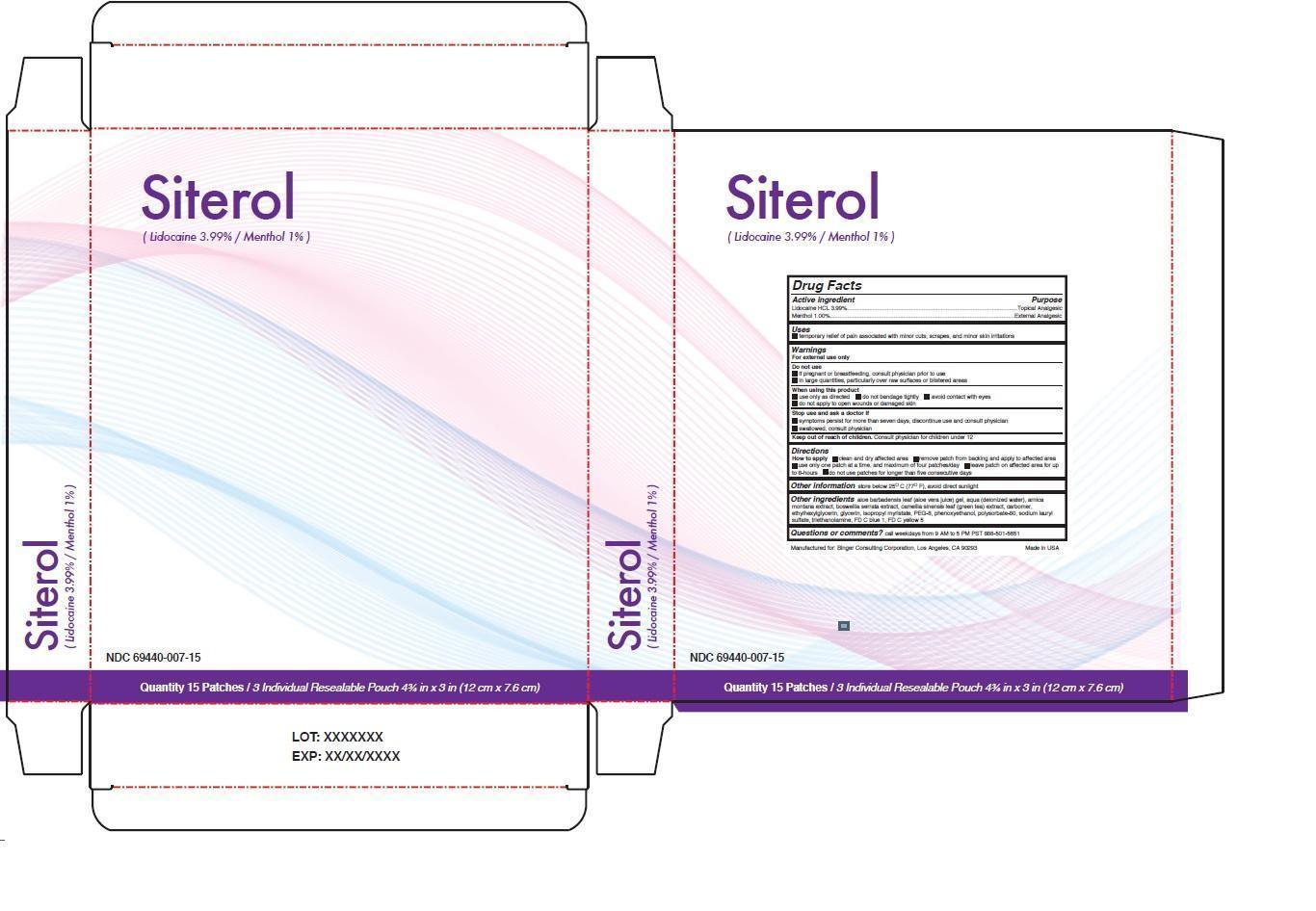
Warnings
For external use only
Do not use
• if pregnant or breastfeeding, consult physician prior to use
• in large quantities, particularly over raw surfaces or blistered areas
When using this product
• use only as directed • do not bandage tightly • avoid contact with eyes
• do not apply to open wounds or damaged skin
Stop use and ask a doctor if
• symptoms persist for more than seven days, discontinue use and consult physician
• swallowed, consult physician
Directions
How to apply • clean and dry affected area • remove patch from backing and apply to affected area • use only one patch at a time, and maximum of four patches/day • leave patch on affected area for upto 8-hours • do not use patches for longer than five consecutive days
Other ingredients aloe barbadensis leaf (aloe vera juice) gel, aqua (deionized water), arnica montana extract, boswellia serrata extract, camellia sinensis leaf (green tea) extract, carbomer, ethylhexylglycerin, glycerin, isopropyl myristate, PEG-8, phenoxyethanol, polysorbate-80, sodium lauryl sulfate, triethanolamine, FD C blue 1, FD C yellow 5