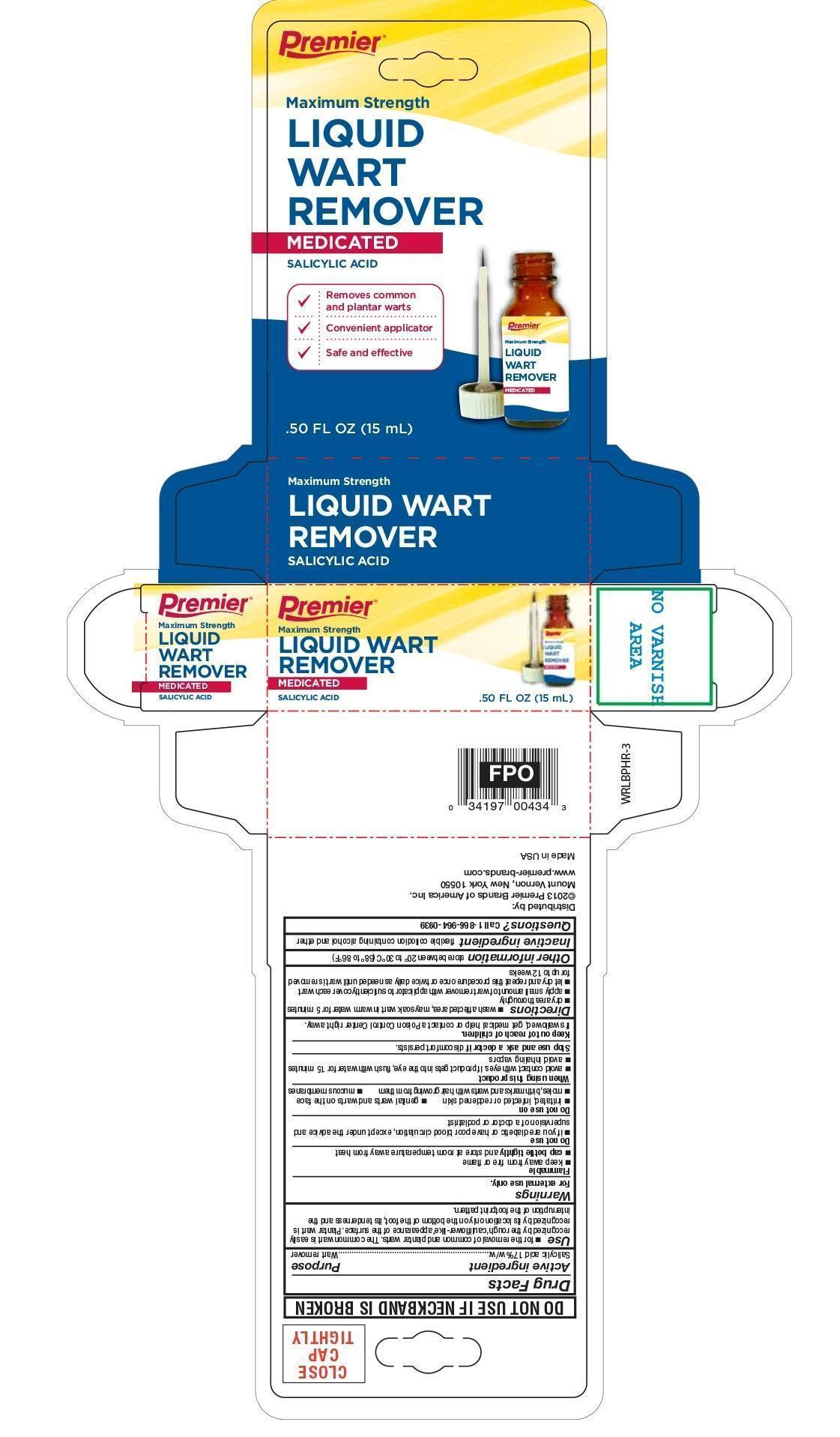
WART REMOVER LIQUID- wart remover liquid liquid
Premier Brands of America Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic acid 17%w/w
Use
- for the removal of common and plantar warts. The common wart is easily recognized by the rough 'cauliflower-like' appearance of the surface. Plantar wart is recognized by its location only on the bottom of the foot, its tenderness and the interruption of the footprint pattern.
Warnings
For external use only.
Flammable
- keep away from fire or flame
-
cap bottle tightly and store at room temperature away heat
Do not use
- if you are a diabetic or have poor blood circulation, except under the advice and supervision of a doctor or podiatrist
Do not use on
- irritated, infected or reddened skin
- genital warts and warts on the face
- moles, birthmarks and warts with hair growing from them
- mucous membranes
When using this product
- avoid contact with eyes. If product gets into the eyes, flush with water for 15 minutes
- avoid inhaling vapors
Stop use and ask doctor if
discomfort persists
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area, may soak wart in warm water for 5 minutes
- dry area thoroughly
- apply small amount of wart remover with applicator to sufficiently cover each wart
- let dry and repeat this procedure once or twice daily until wart is removed for up to 12 weeks
Other information
store between 20°C to 30°C (68°F to 86°F)
Inactive ingredients
camphor, castro oil, ethanol, ethyl ether, nitrocellulose
Questions?
Call 1-866-964-0939
Principal Display Panel
Premier Brands
Maximum Strength
Liquid Wart Remover
Medicated
Salicylic Acid
Removes common and plantar warts
Convenient Applicator
Safe and Effective
0.50 FL OZ (15 mL)
Premier Brands of America Inc.
