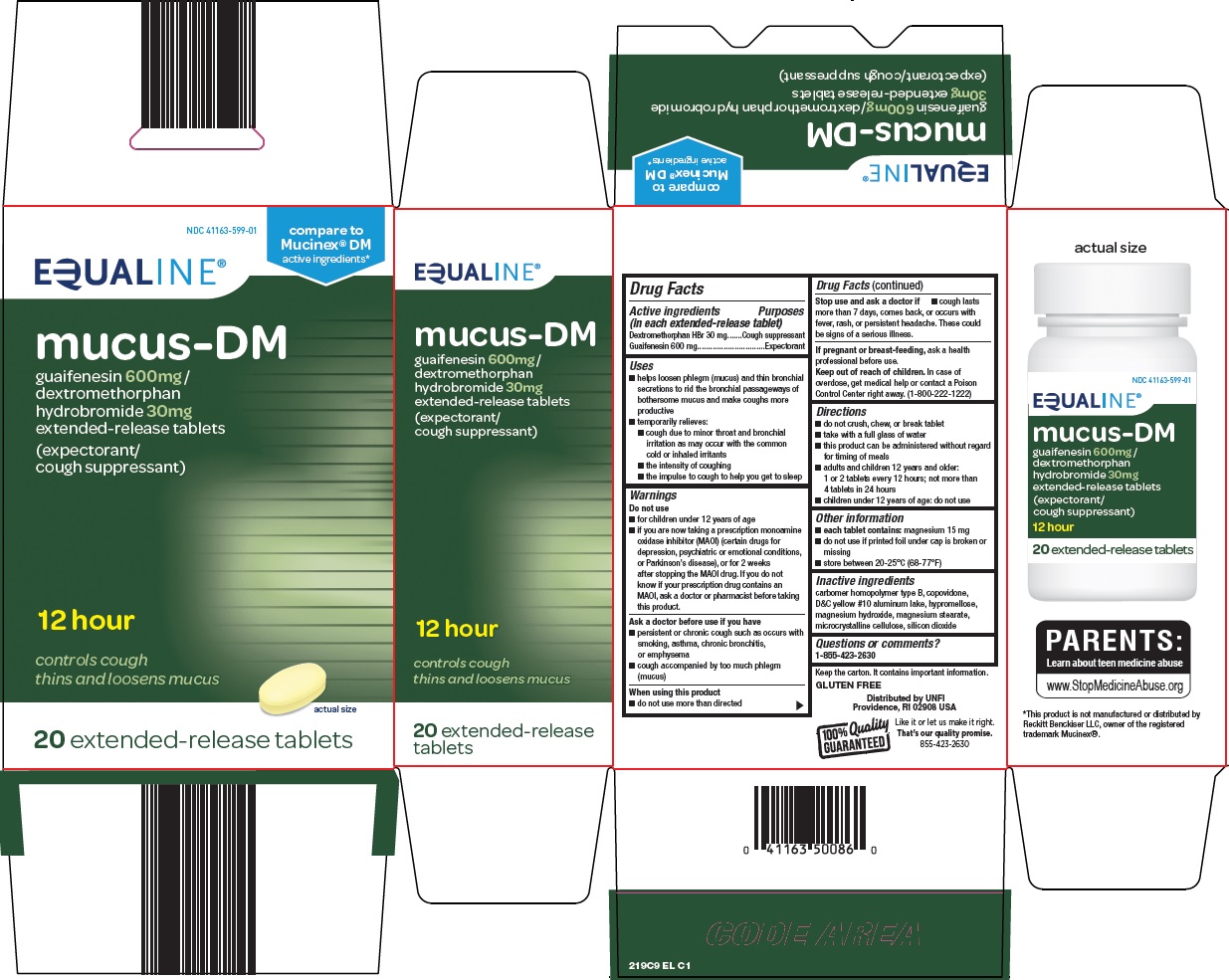
Uses
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- •
- temporarily relieves:
- •
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- •
- the intensity of coughing
- •
- the impulse to cough to help you get to sleep
Warnings
Do not use
- •
- for children under 12 years of age
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
Directions
- •
- do not crush, chew, or break tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for timing of meals
- •
- adults and children 12 years and older:
1 or 2 tablets every 12 hours; not more than 4 tablets in 24 hours
- •
- children under 12 years of age: do not use
Other information
- •
- each tablet contains: magnesium 15 mg
- •
- Do not use if printed foil under cap is broken or missing
- •
- store between 20-25°C (68-77°F)