ACETAMINOPHEN 500 mg & DIPHENHYDRAMINE HCl 25 mg
RAPID RELEASE GELCAPS
Each Rapid Release Gelcap Contains:
Acetaminophen USP 500 mg and Diphenhydramine Hydrochloride USP 25 mg
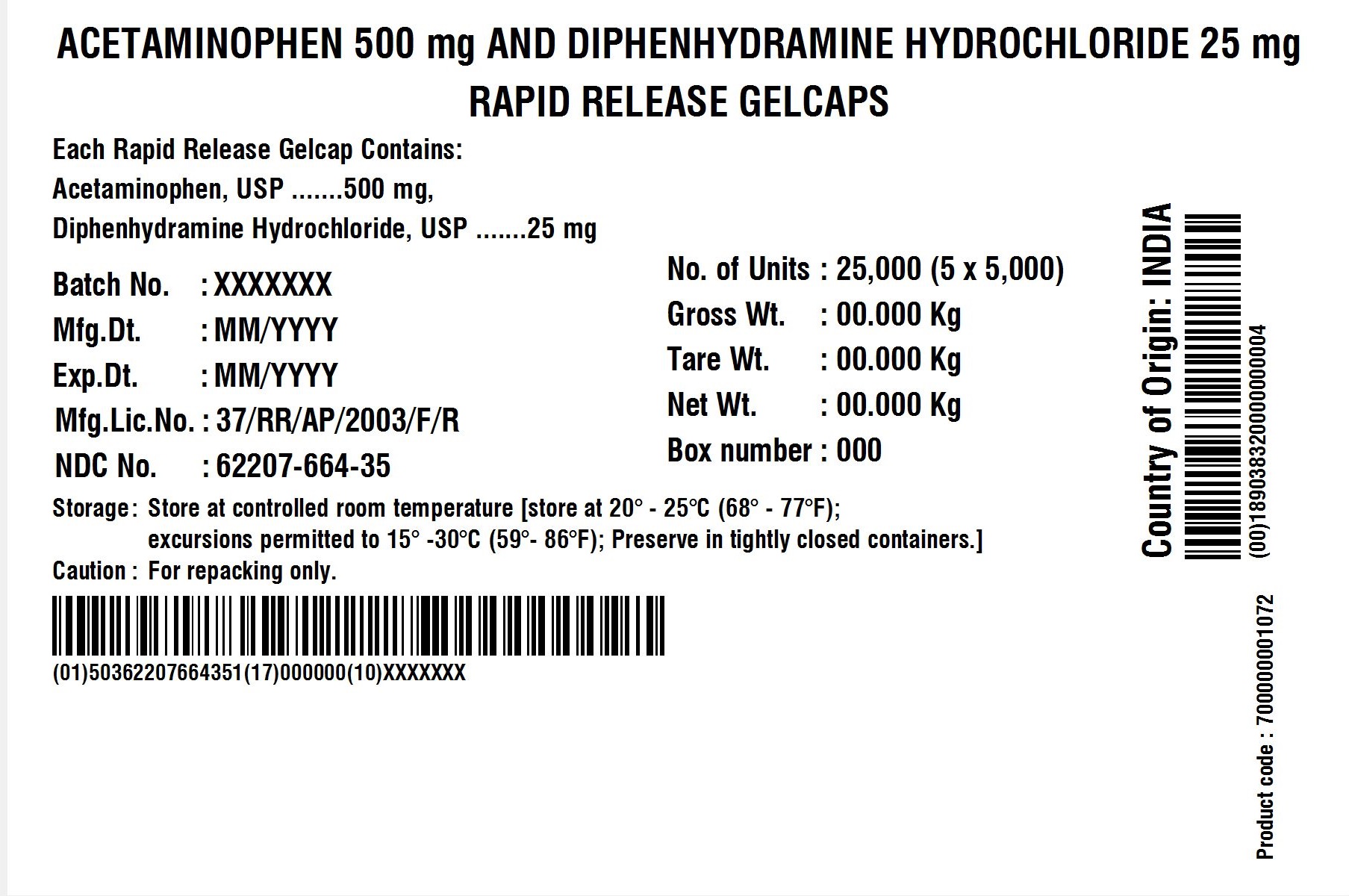
BOX LABEL
| Batch No.: | XXX | No. of Units | 5 x 5000 |
| Mfg. Dt.: | MMM/YYYY | Gross Wt.: | 0.00 kg |
| Exp. Dt.: | MMM/YYYY | Tare Wt:. | 0.00 kg |
| Mfg. Lic. No.: | 37/RR/AP/2003/F/R | Net Wt.: | 0.00 kg |
| NDC No. | XXXXX-XXX-XX | Box Number: | 000 |
| Storage: | Store at 20 – 25C (68 – 77F); excursions permitted to 15 – 30 C (59 – 86F); Preserve in tightly closed containers. | ||
| Caution | For Repacking only. | ||
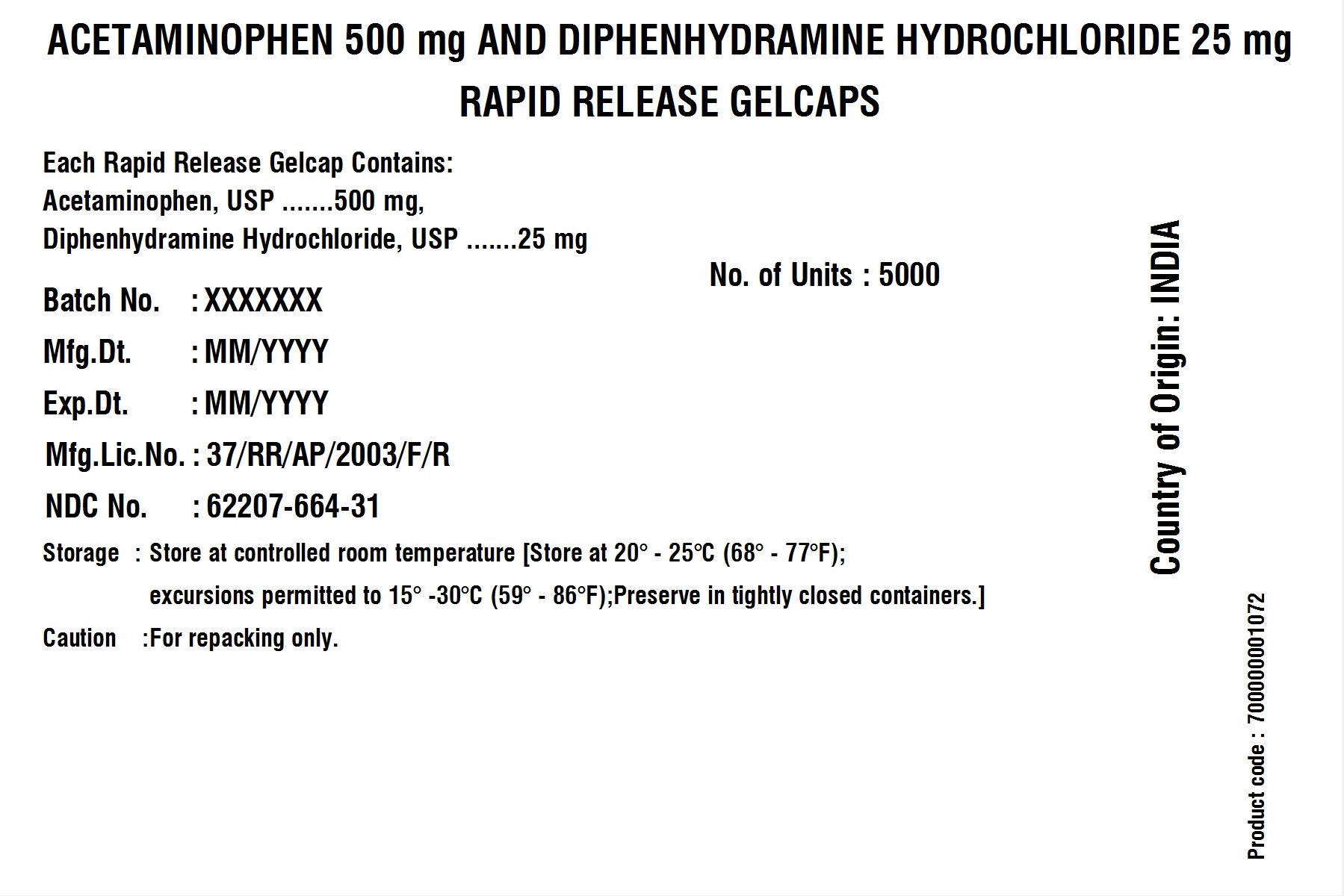
POUCH LABEL
| Batch No.: | XXX | No. of Units | 5000 |
| Mfg. Dt.: | MMM/YYYY | ||
| Exp. Dt.: | MMM/YYYY | ||
| Mfg. Lic. No.: | 37/RR/AP/2003/F/R | ||
| NDC No. | XXXXX-XXX-XX | ||
| Storage: | Store at 20 – 25C (68 – 77F); excursions permitted to 15 – 30 C (59 – 86F); Preserve in tightly closed containers. | ||
| Caution | For Repacking only. | ||
Gil/PL/041
Product Code: XXXXXXX
Manufactured in India by:
Granules India Limited
Plot No. 160/A, 161/E, Gagillapur Village
Qutbullapur Mandal, Ranga Reddy Dt – 500 043, AP.