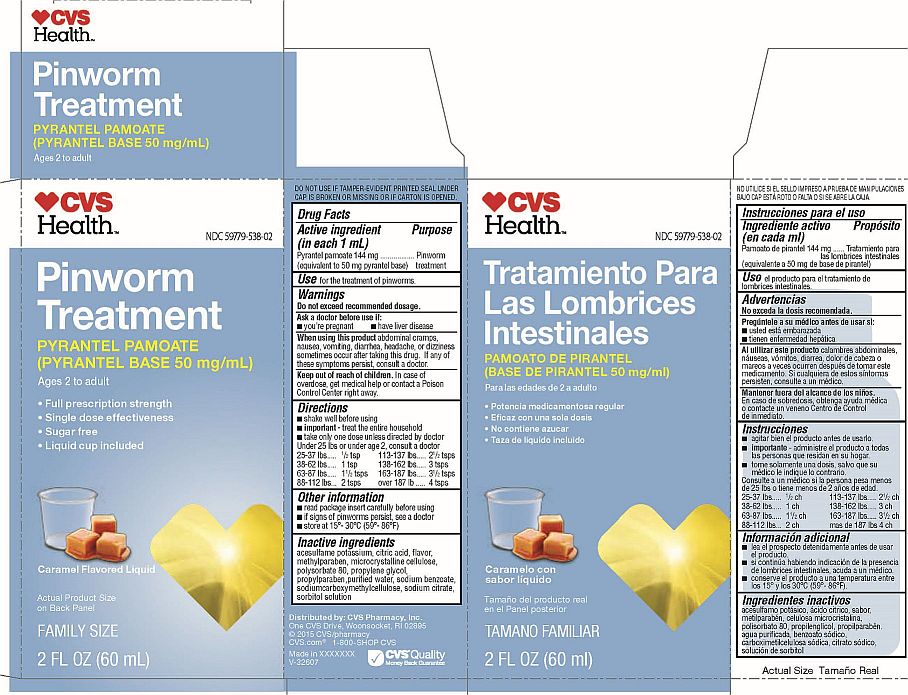
Pinworm Treatment
NDC 59779-538-02
PYRANTEL PAMOATE
(PYRANTEL BASE 50 mg/mL)
Ages 2 to Adult
- Full prescription strength
- Single dose effectiveness
- Sugar free
- Liquid cup included
Caramel Flavored Liquid
Actual Product Size on Back Panel
FAMILY SIZE
2 FL OZ (60 mL)
When using this product abdominal cramps, nausea, vomiting, diarrhea, headache, or dizziness sometimes occur after taking this drug. If any of these symptoms persist, consult a doctor.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- shake well before using
- important - treat the entire household
- take only one dose unless directed by a doctor
Under 25 lbs or under age 2, consult a doctor
25-37 lbs..... 1/ 2 tsp
36-62 lbs.....1 tsp
63-87 lbs.....1 1/ 2 tsps
88-112 lbs....2 tsps
113-137 lbs.....2 1/ 2 tsps
138-162 lbs.....3 tsps
163-187 lbs.....3 1/ 2 tsps
over 187 lbs.....4 tsps
Other information
- read package insert carefully before using
- if signs of pinworms persist, see a doctor
Inactive ingredients
acesulfame potassium, citric acid, flavor, methylparaben, microcrystalline cellulose, polysorbate 80, propylene glycol, propylparaben, purified water, sodium benzoate, sodiumcarboxymethylcellulose, sodium citrate, sorbitol solution