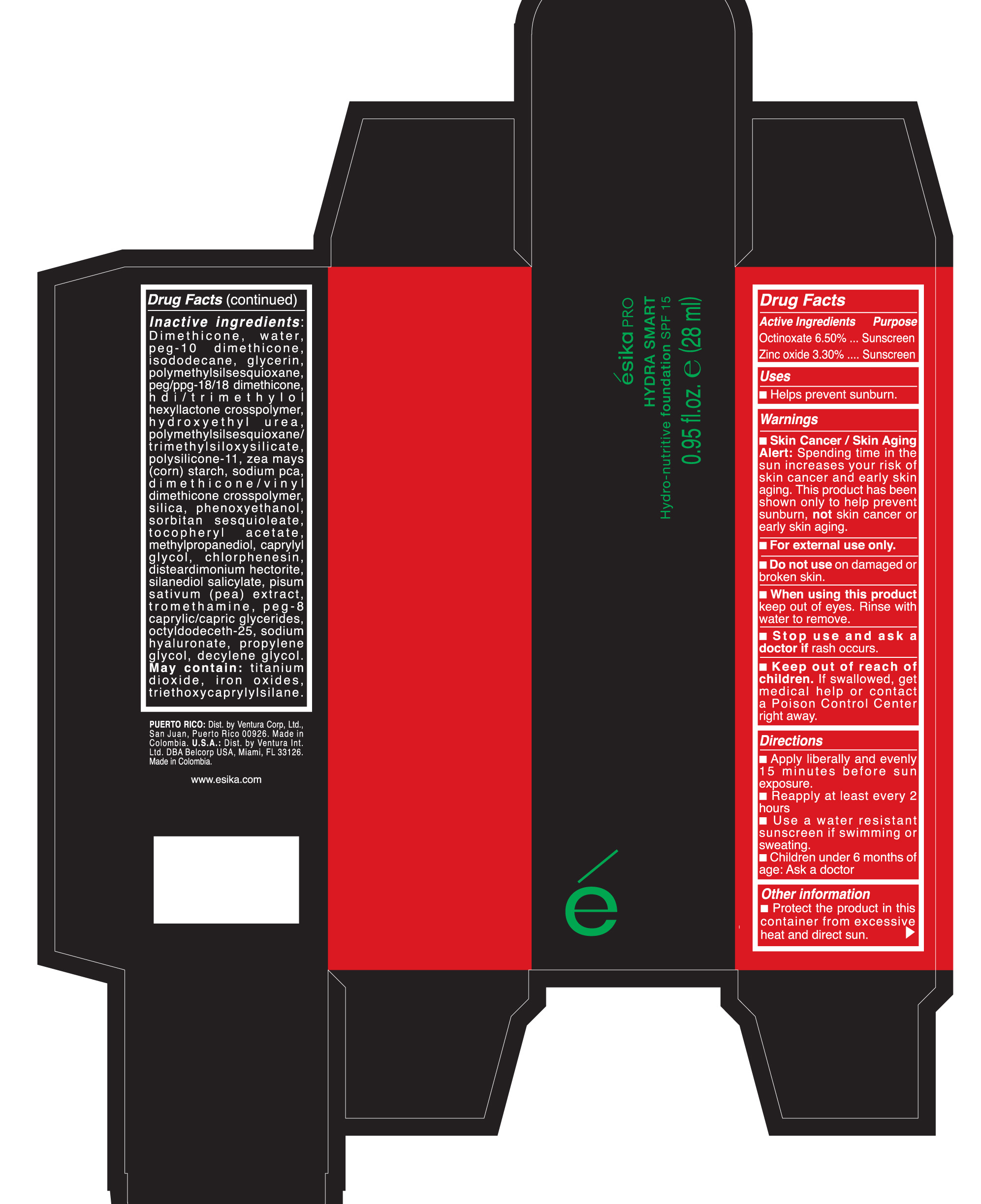
Active Ingredients Purpose
Avobezone 1.80% Sunscreen
Octocrylene 4.5% Sunscreen
Octisalate 4.5% Sunscreen
Keep out of reach of children. If product is swallowed, get medical help or
contact a Poison Control Center right away.
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help sunburn, not skin cancer or early skin aging.
For external use only.
When using this product keep out of eyes. Rinse with water to remove
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months of age: Ask a doctor.
Inactive ingredients:
Dimethicone, water, PEG-10 dimethicone, isododecane, glycerin, polymethylsilsesquioxane, peg/ppg-18/18 dimethicone, hdi/trimethylol hexyllactone crosspolymer, hydroxyethyl urea, polymethylsilsesquioxane/trimethylsiloxysilicate, polysilicone-11, Zea Mays (corn) starch, sodium pca, dimethicone/vinyl dimethicone crosspolymer, silica, phenoxyethanol, sorbitan sesquioleate, tocopheryl acetate, methylpropanediol, caprylyl glycol, chlorphenesin, disteardimonium hectorite, silanediol salicylate, pisum sativum (pea) extract extract, tromethamine, peg-8 caprylic/capric glycerides, octyldodeceth-25, sodium hyaluronate, propylene glycol, decylene.
May contain titanium, iron oxides, triethoxycaprylylsilane