Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- do not wear a contact lens if your eye is red
Stop use and ask a doctor if you experience:
- eye pain
- changes in vision
- increased redness of the eye
- itching worsens or lasts for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
adults and children 2 years of age and older:
- put 1 drop in the affected eye(s) once daily, no more than once per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
- children under 2 years of age: consult a doctor
Inactive ingredients
benzalkonium chloride 0.01%, dibasic sodium phosphate, edetate disodium, hydrochloric acid/sodium hydroxide (adjust pH), povidone, purified water, and sodium chloride
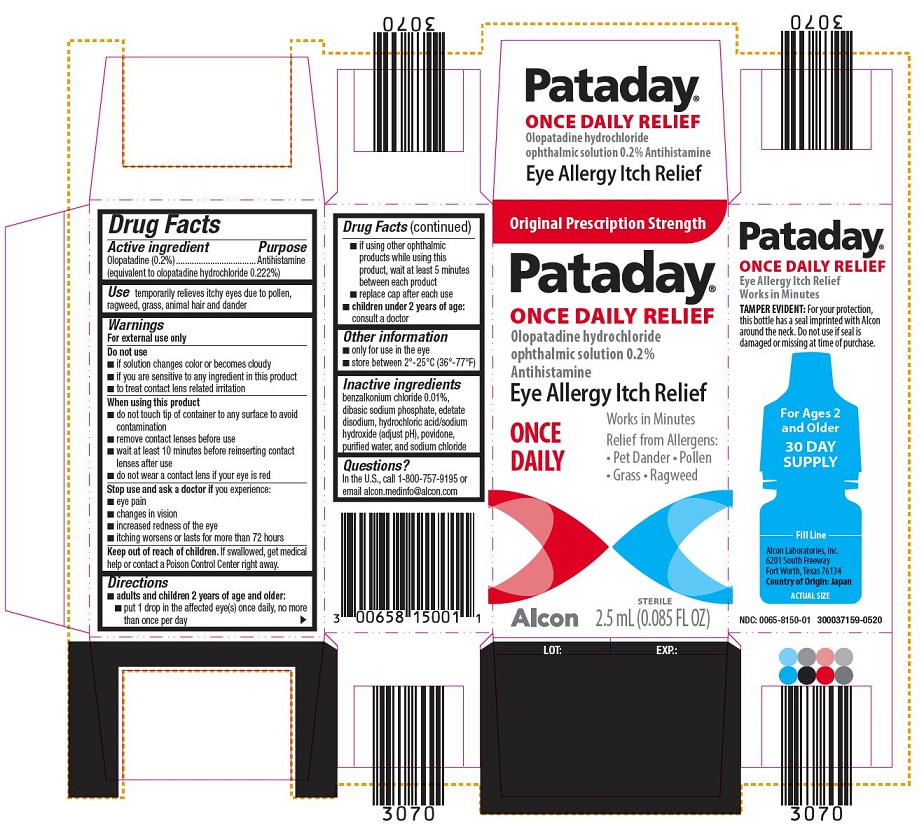
PRINCIPAL DISPLAY PANEL
Pataday®
ONCE DAILY RELIEF
Olopatadine hydrochloride
ophthalmic solution 0.2% Antihistamine
2.5 mL (0.085 FL OZ)
STERILE
EYE ALLERGY ITCH RELIEF
Only for use in the eye. Store between 2°– 25° C (36°– 77° F)
TAMPER EVIDENT: For your protection, this bottle has a seal imprinted with Alcon around the neck. Do not use if seal is damaged or missing at time of purchase.
Alcon Laboratories, Inc.
Fort Worth, TX 76134
LOT: EXP.:
H15725-219

Original Prescription Strength
Pataday
ONCE DAILY RELIEF
Olopatadine hydrochloride
ophthalmic solution 0.2%
Antihistamine
Eye Allergy Itch Relief
ONCE DAILY
Works in Minutes Relief from Allergens:
- Pet Dander
- Pollen
- Grass
- Ragweed
STERILE
2.5 mL (0.085 FL OZ)
Alcon
Pataday
ONCE DAILY RELIEF
Eye Allergy Itch Relief
Works in Minutes
TAMPER EVIDENT: For your protection,
this bottle has a seal imprinted with Alcon
around the neck. Do not use if seal is
damaged or missing at time of purchase.
For Ages 2
and Older
30 DAY
SUPPLY
________Fill Line________
Alcon Laboratories, Inc.
6201 South Freeway
Fort Worth, Texas 76134
Country of Origin: Japan
ACTUAL SIZE
NDC: 0065-6150-01 300037159-0520