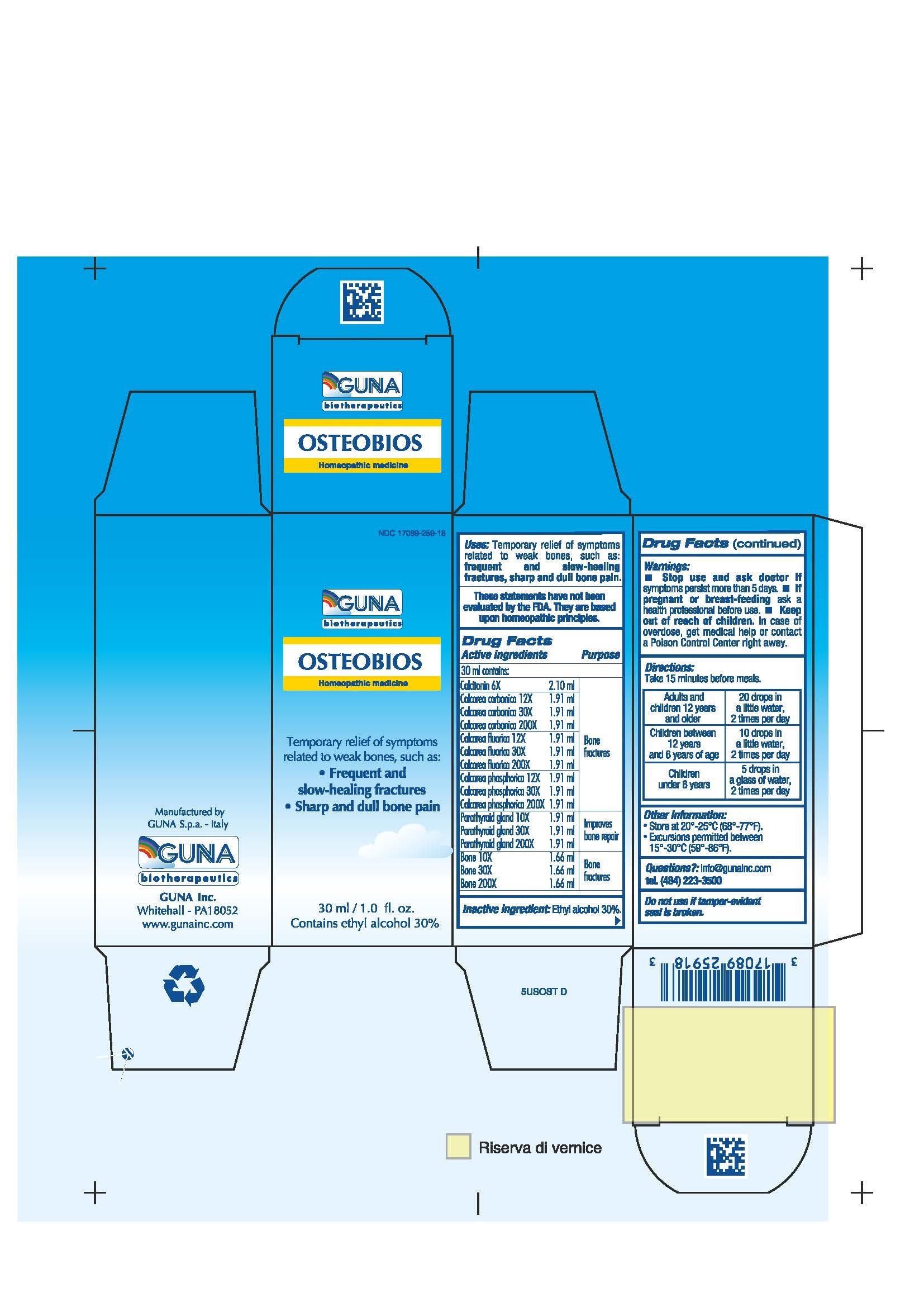
ACTIVE INGREDIENTS/PURPOSE
BONE 10X 30X 200X BONE FRACTURES
CALCAREA FLUORICA 12X 30X 200X BONE FRACTURES
CALCAREA CARBONICA 12X 30X 200X BONE FRACTURES
CALCAREA PHOSPHORICA 12X 30X 200X BONE FRACTURES
CALCITONIN 6X BONE FRACTURES
PARATHYROID GLAND 10X 30X 200X IMPROVES BONE REPAIR
USES
Temporary relief of symptoms related weak bones such as:
- Frequent and slow-healing fractures
-
Sharp and dull bone pain
WARNINGS
Stop use and ask doctor if symptoms persist more than 5 days.
If pregnant or breast-feeding ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Contains ethyl alcohol 30%