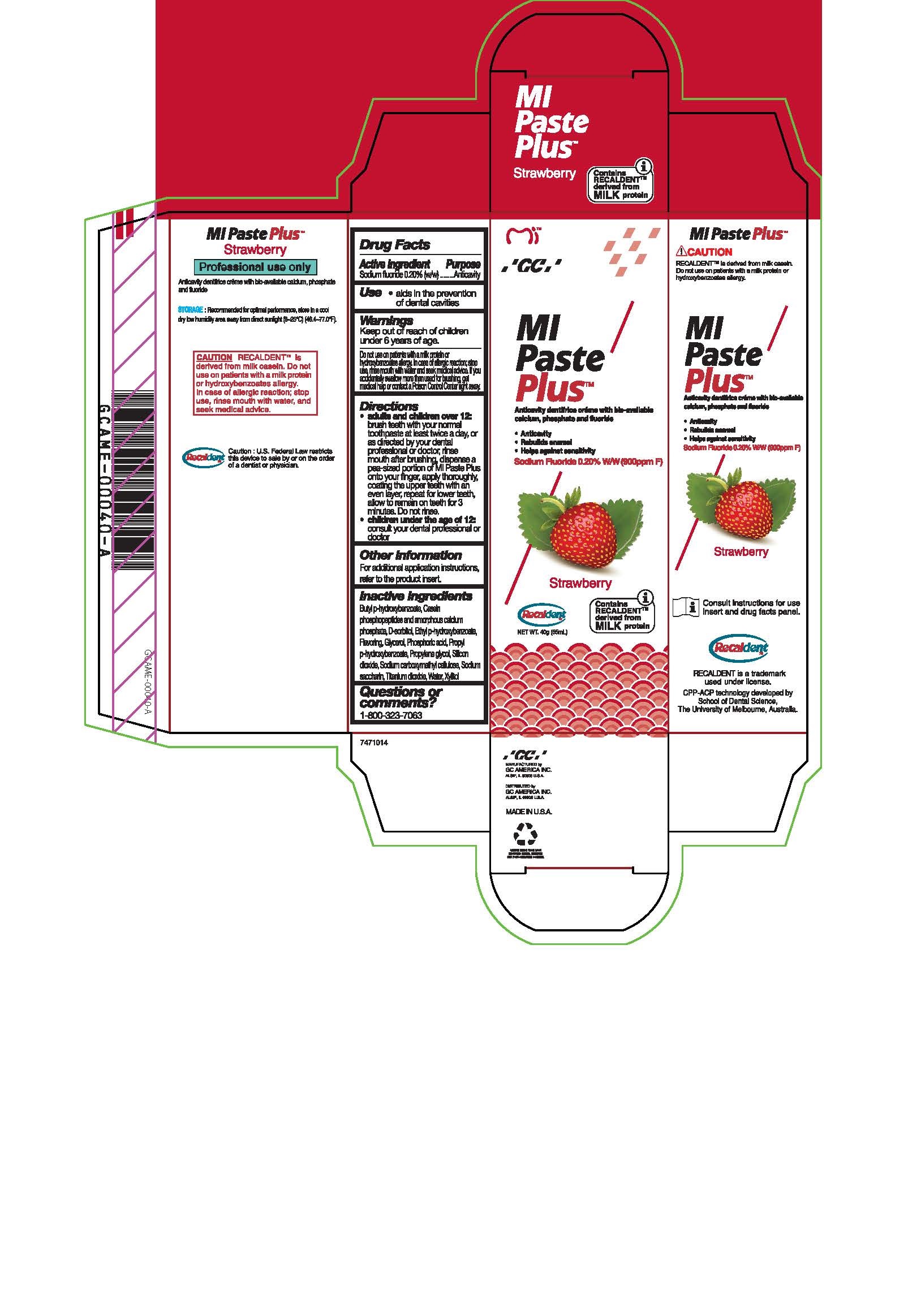
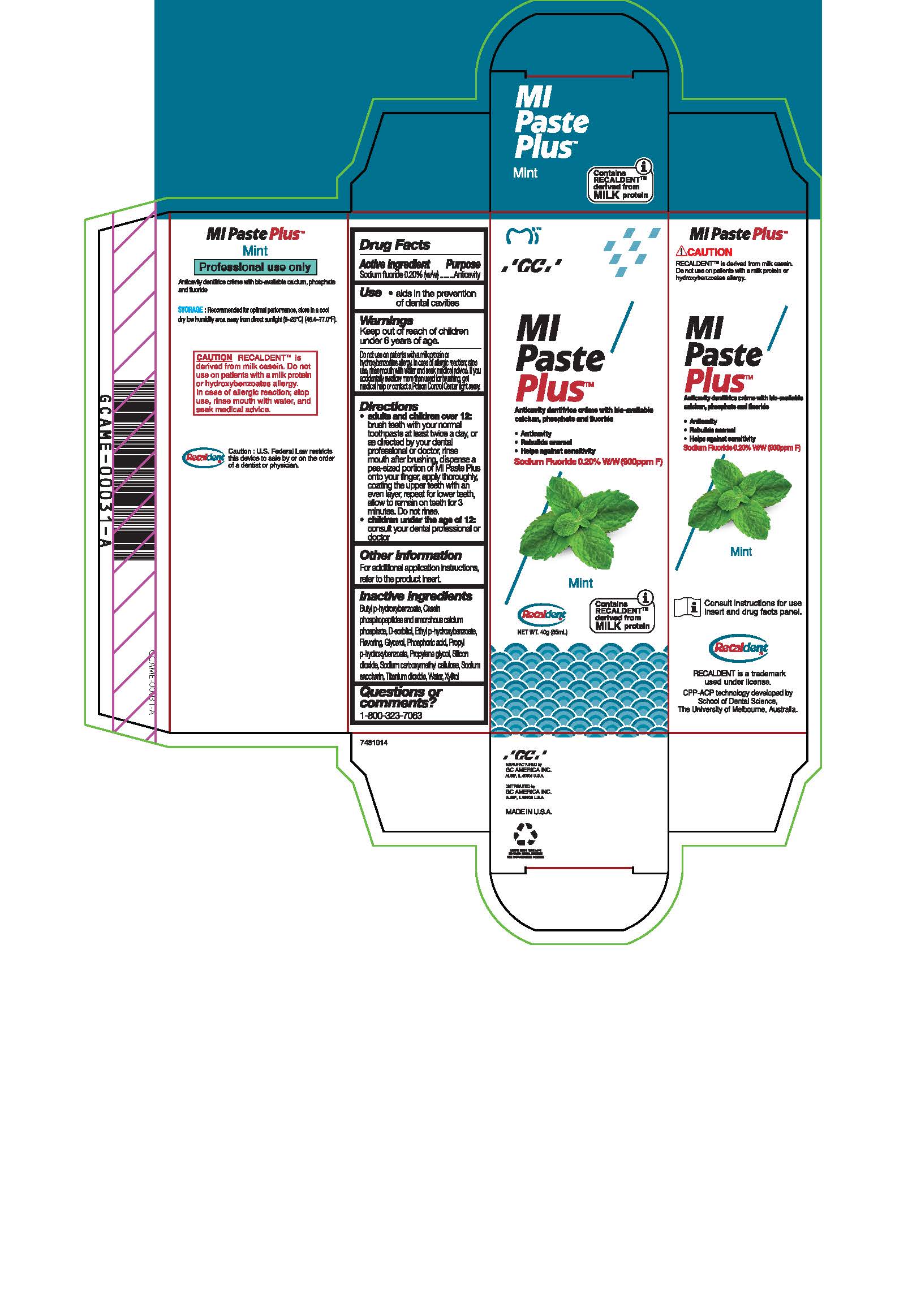
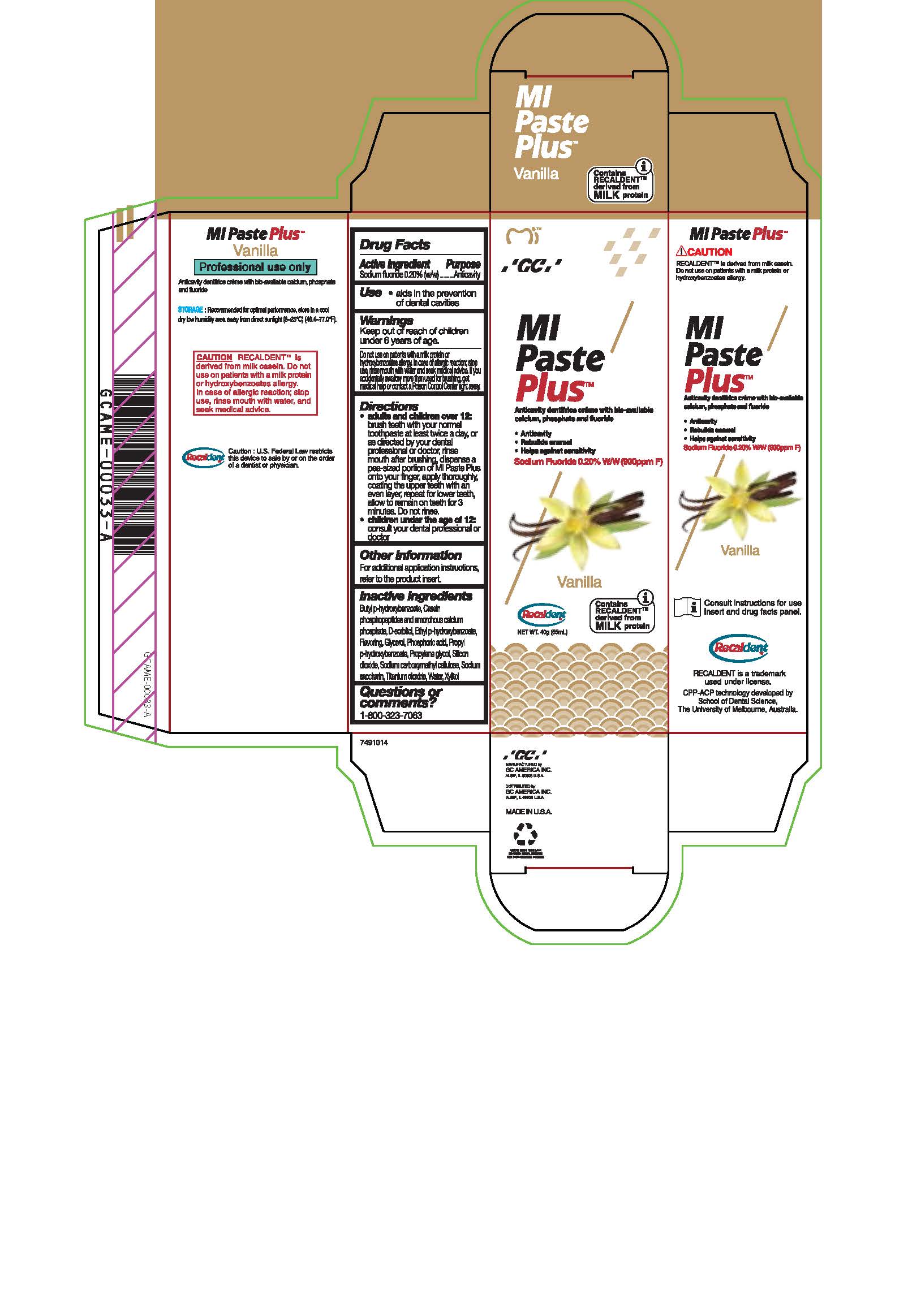
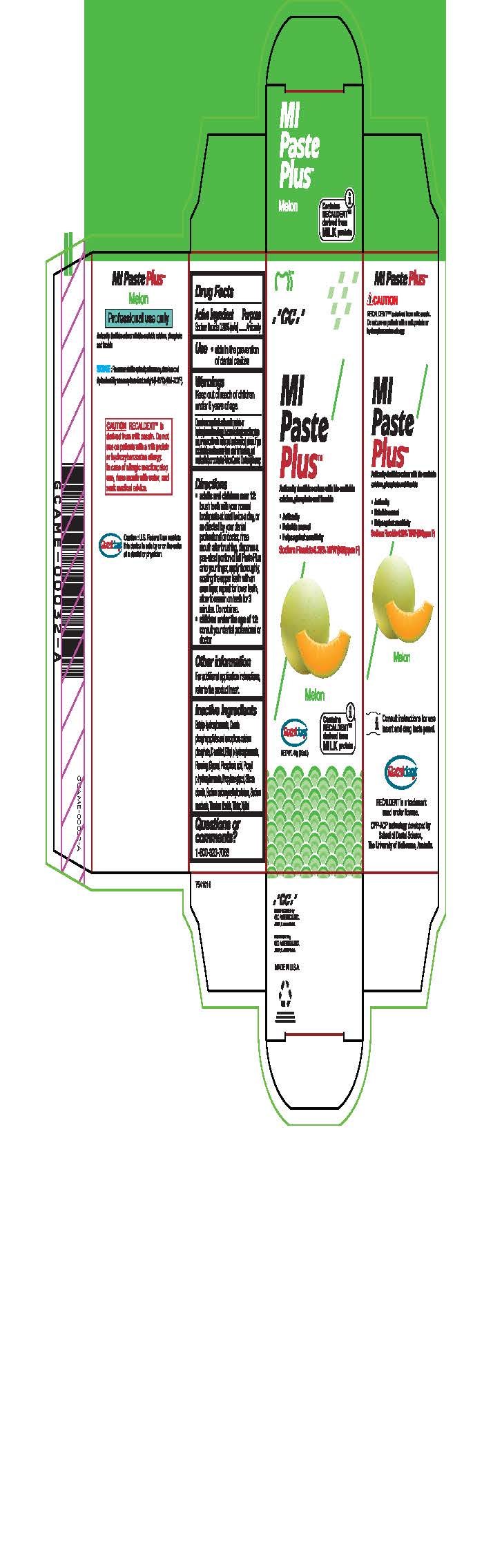
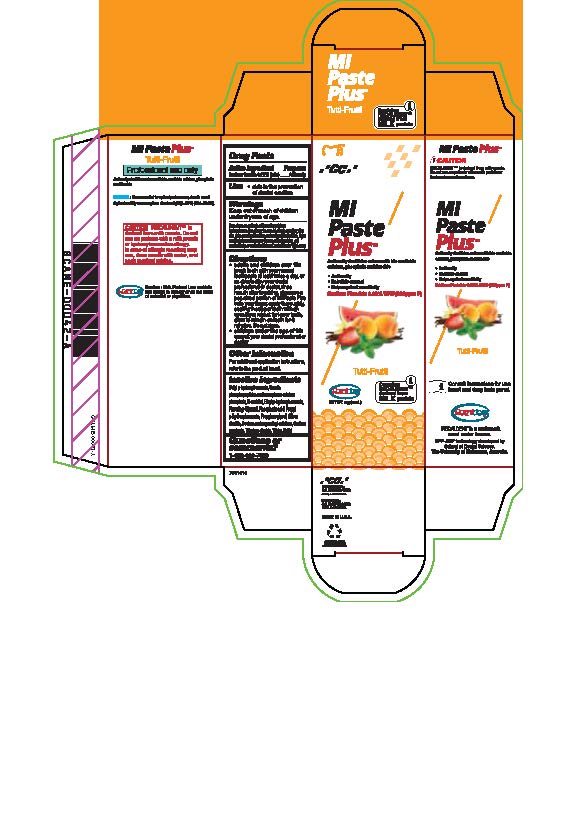
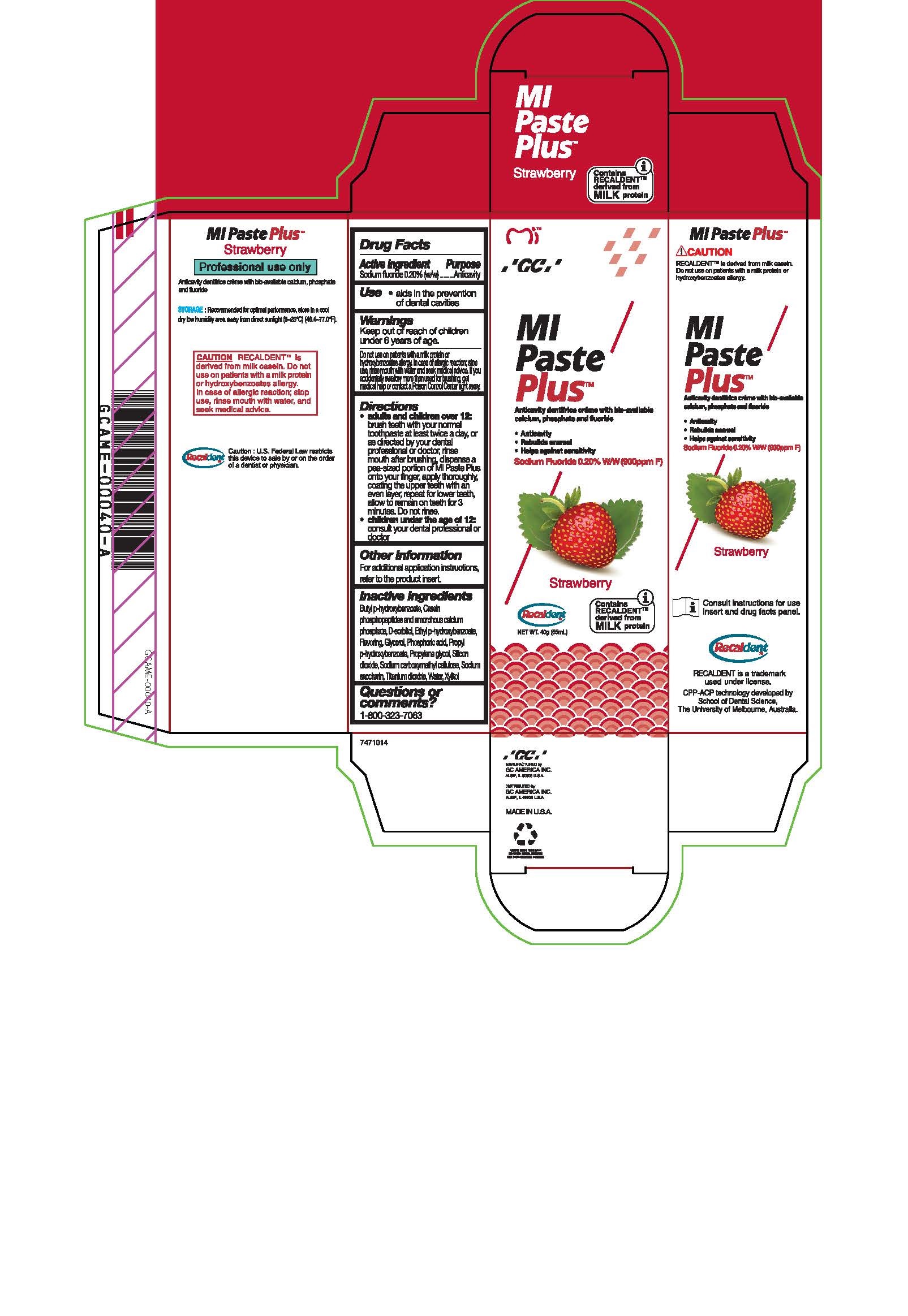
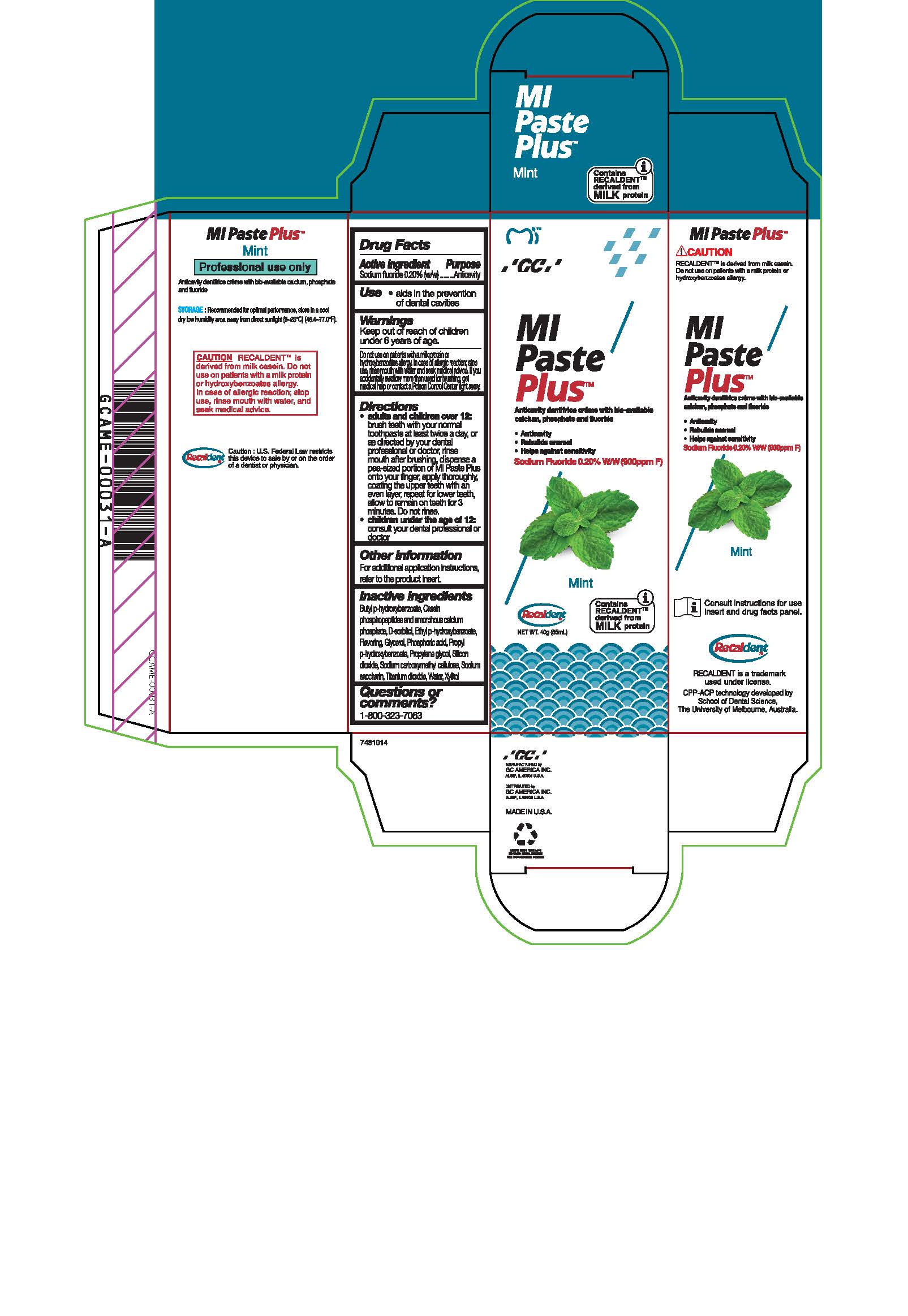
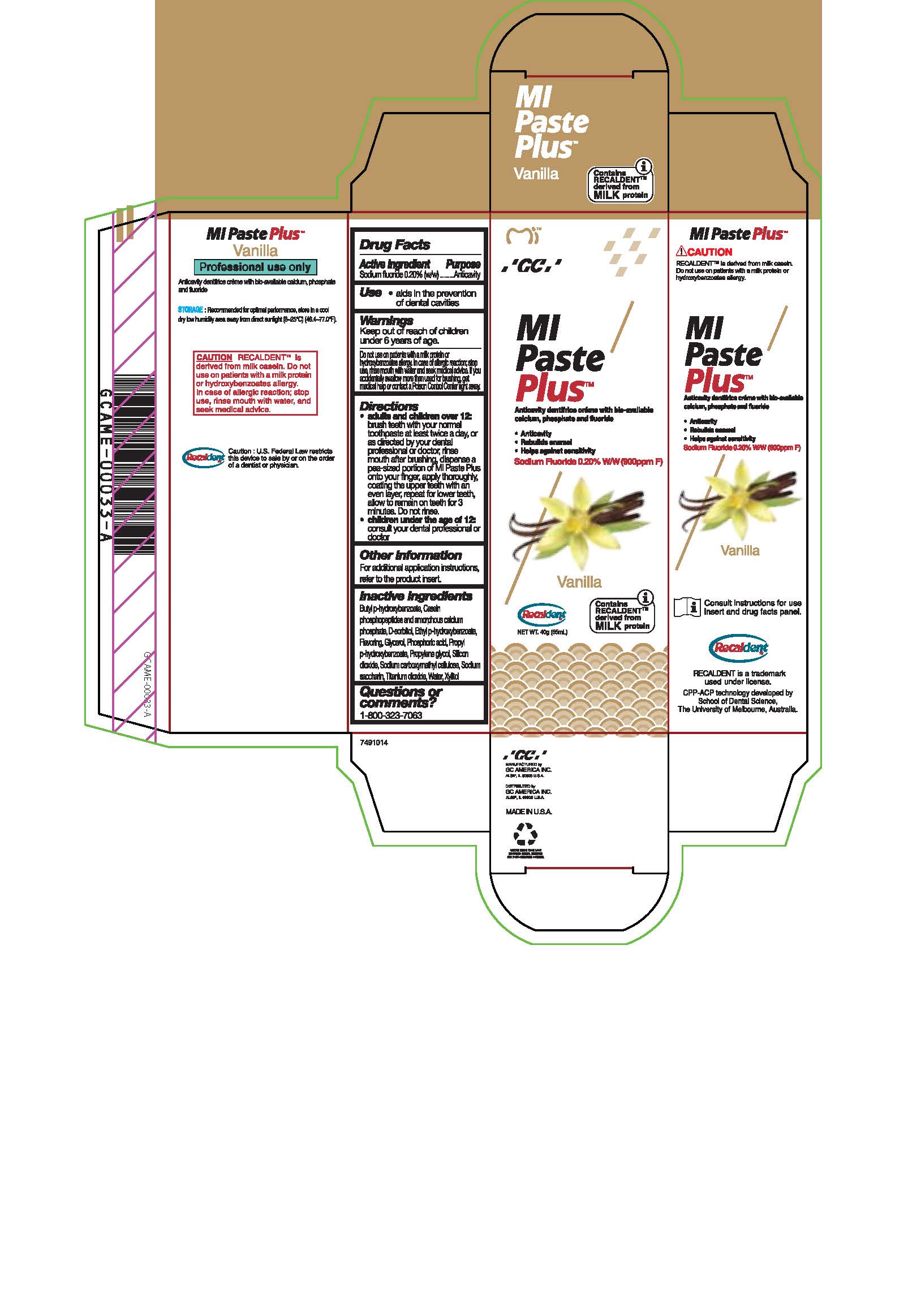
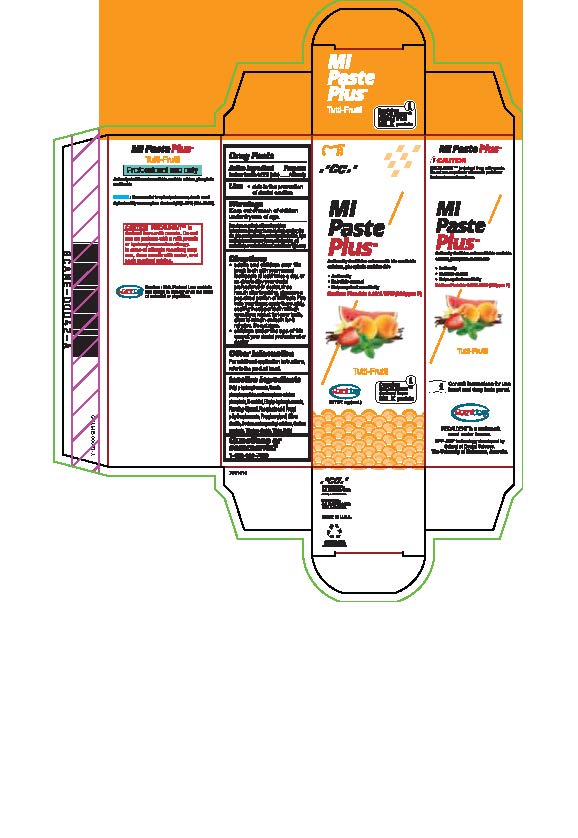
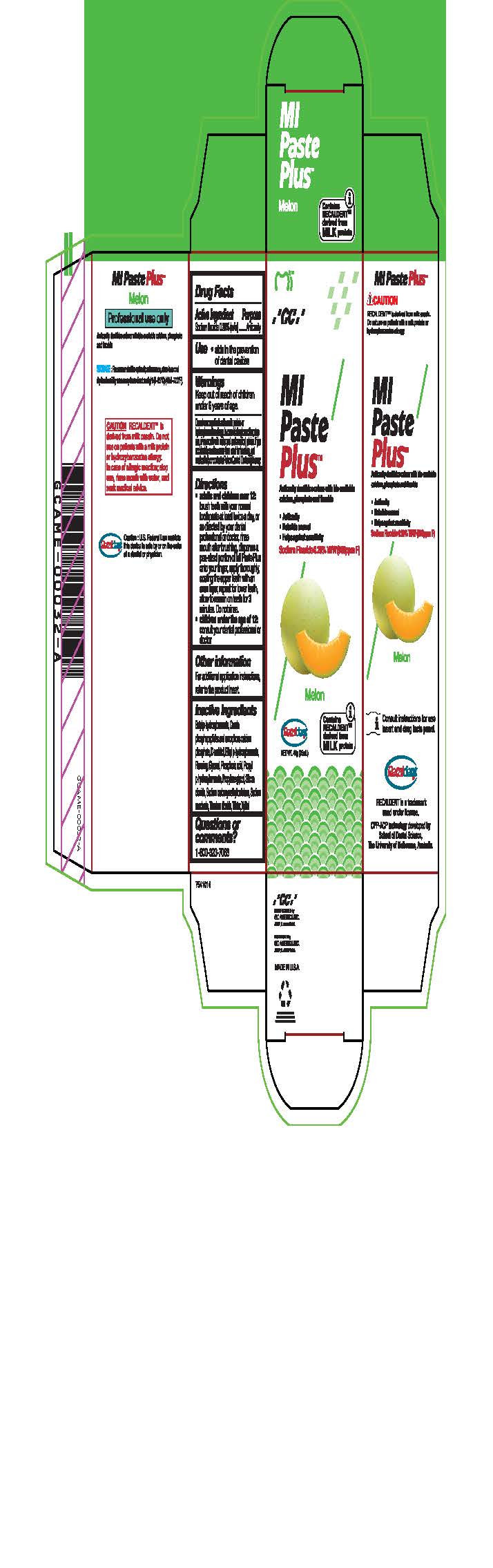
Warnings
Do not use on patients with a milk protein or hydroxybenzoates allergy. In case of allergic reaction, stop use, rinse mouth with water and seek medical advice. If you accidentally swallow more than used for brushing, get medical help or contact a Poison Control Center right away.
Directions
* Adults and children over 12: brush teeth with your normal toothpaste at least twice a day, or as directed by your dental profession or doctor, rinse mouth after brushing, dispense a pea-sized portion of MI Paste Plus onto your finger, apply thoroughly, coating the upper teeth with an even layer, repeat for lower teeth, allow to remain on teeth for 3 mintues. Do not rinse.
* Children under the age of 12: consult your dental professional or doctor.
Inactive ingredients
Butyl p-hydroxybenzoate, Casein phosphopeptides and amorphous calcium phosphate, D-sorbitol, Ethyl p-hydroxybenzoate, Flavoring, Glycerol, Phosphoric acid, Propyl p-hydroxybenzoate, Propylene glycol, Silicon dioxide, sodium carboxymethyl cellulose, Sodium saccharin, Titanium dioxide, Water, Xylitol