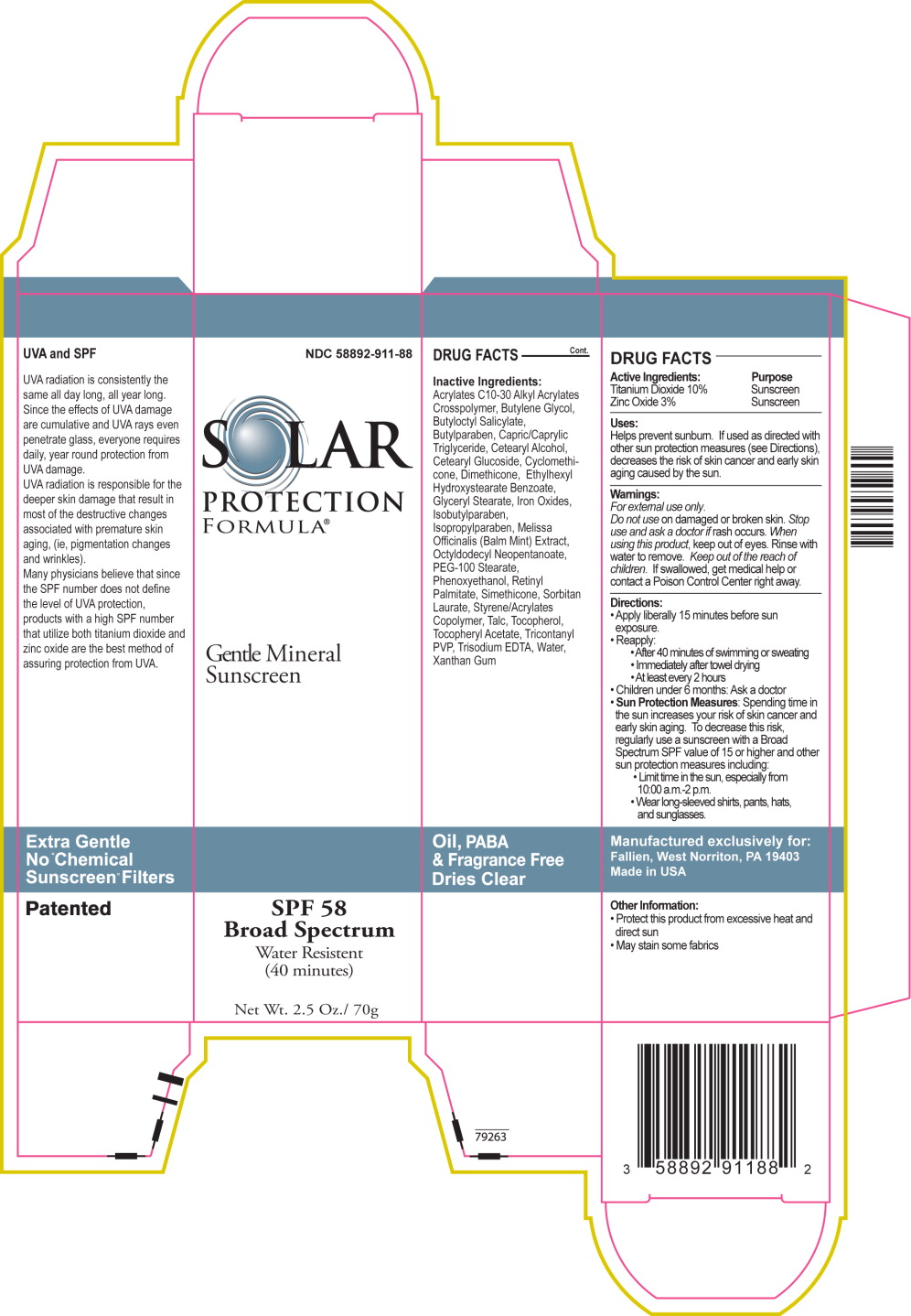
Uses:
Helps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions:
- Apply liberally 15 minutes before sun exposure.
- Reapply:
- After 40 minutes of swimming or sweating
- Immediately after towel drying
- At least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10:00 a.m.-2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
Inactive Ingredients:
Acrylates C10-30 Alkyl Acrylates Crosspolymer, Butylene Glycol, Butyloctyl Salicylate, Butylparaben, Capric/Caprylic Triglyceride, Cetearyl Alcohol, Cetearyl Glucoside, Cyclomethicone, Dimethicone, Ethylhexyl Hydroxystearate Benzoate, Glyceryl Stearate, Iron Oxides, Isobutylparaben, Isopropylparaben, Melissa Officinalis (Balm Mint) Extract, Octyldodecyl Neopentanoate, PEG-100 Stearate, Phenoxyethanol, Retinyl Palmitate, Simethicone, Sorbitan Laurate, Styrene/Acrylates Copolymer, Talc, Tocopherol, Tocopheryl Acetate, Tricontanyl PVP, Trisodium EDTA, Water, Xanthan Gum