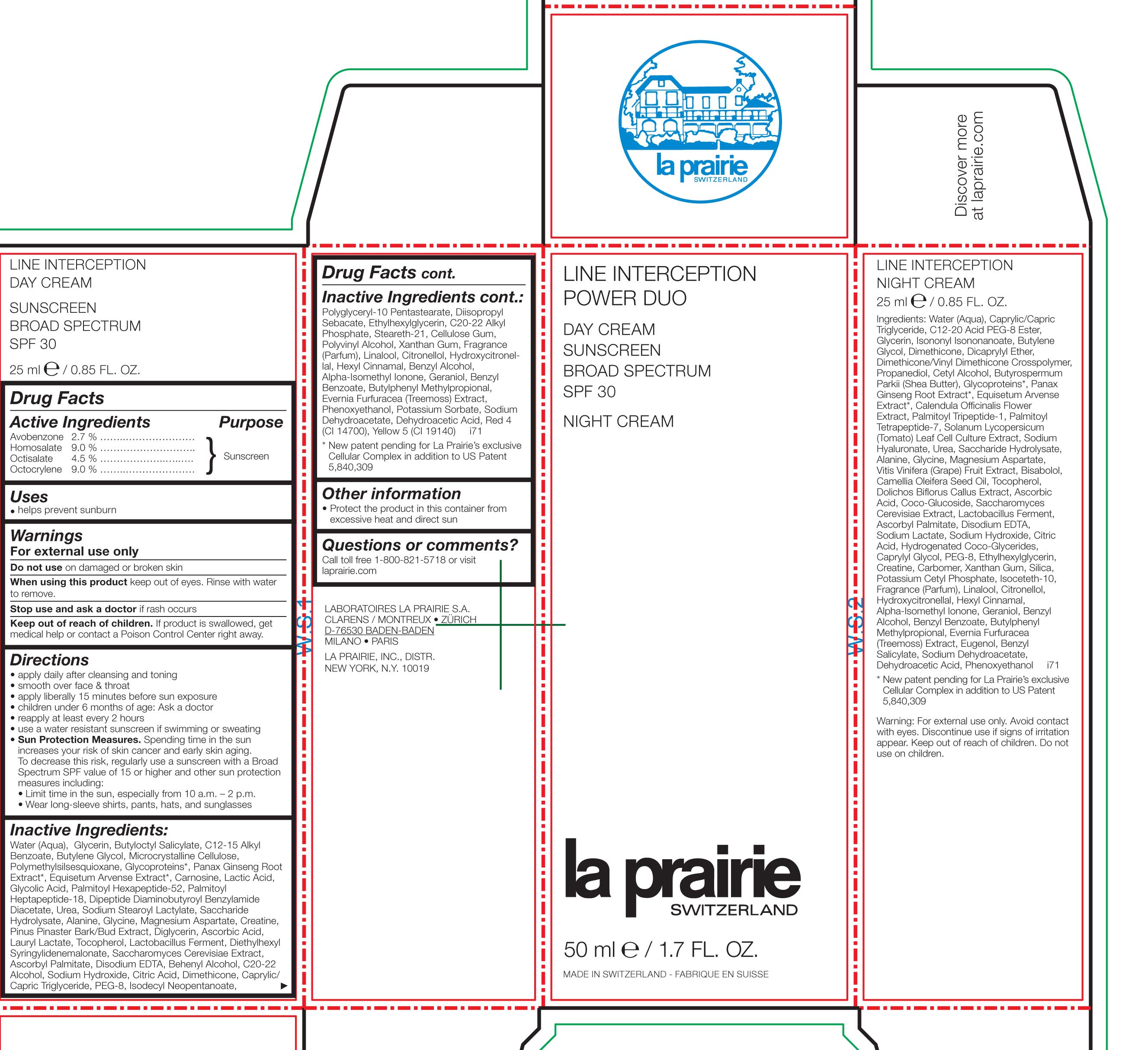
Active Ingredients Purpose
Avobenzone 2.7% .............
Homosalate 9.0% ............. Sunscreen
Octisalate 4.5% ............
Octocrylene 9.0% ...........
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove
Directions
- apply daily after cleansing and toning
- smooth over face & throat
- apply liberally 15 minutes before sun exposure
- children und 6 months of age: Ask a doctor
- reapply at least every 2 hours
- use a water resistan sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sunincreases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and oher sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
Inactive Ingredients:
Water (Aqua), Glycerin, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Butylene Glycol, Microcrystalline Cellulose, Polymethylsilsquioxane, Glycoproteins, Panax Ginseng Root Extract, Equisetum Arvense Extract, Carnosine, Lactic Acid, Glycolic Acid, Palmitoyl Hexapeptide-52, Palmitoyl Heptapeptide-18, Dipeptide Diaminobutyroyl Benzylamide Diacetate, Urea, Sodium Stearoyl Lactylate, Saccharide Hydrolysate, Alanine, Glycine, Magnesium Aspartate, Creatine, Pinus Pinaster Bark/Bud Extract, Diglycerin, Ascorbic Acid, Lauryl Lactate, Tocopherol, Lactobaillus Ferment, Diethylhexyl Syringylidenemalonate, Saccharomyces Cerevisiae Extract, Ascorbyl Palmitate, Disodium EDTA, Behenyl Alcohol, C20-22 Alcohol, Sodium Hydroxide, Citric Acid, Dimethicone, Caprylic/Capric Triglyceride, PEG-8, Isodecyl Neopentanoate, Polyglyceryl-10 Pentastearate, Diisopropyl Sebacate, Ethylhexylglycerin, C20-22 Alkyl Phosphate, Steareth-21, Cellulose Gum, Polyvinyl Alcohol, Xanthan Gum, Fragrance (Parfum) Linalool, Citronellol, Hydroxycitronellal, Hexyl Cinnamal, Benzyl Benzoate, Butylphenyl Methylpropional, Evernia Furfuracea (Treemoss) Extract, Phenoxyethanol, Potassium Sorbate, Sodium Dehydroacetate, Dehydroacetic Acid, Reg 4 (CI 14700), Yellow 5 (CI 19140)