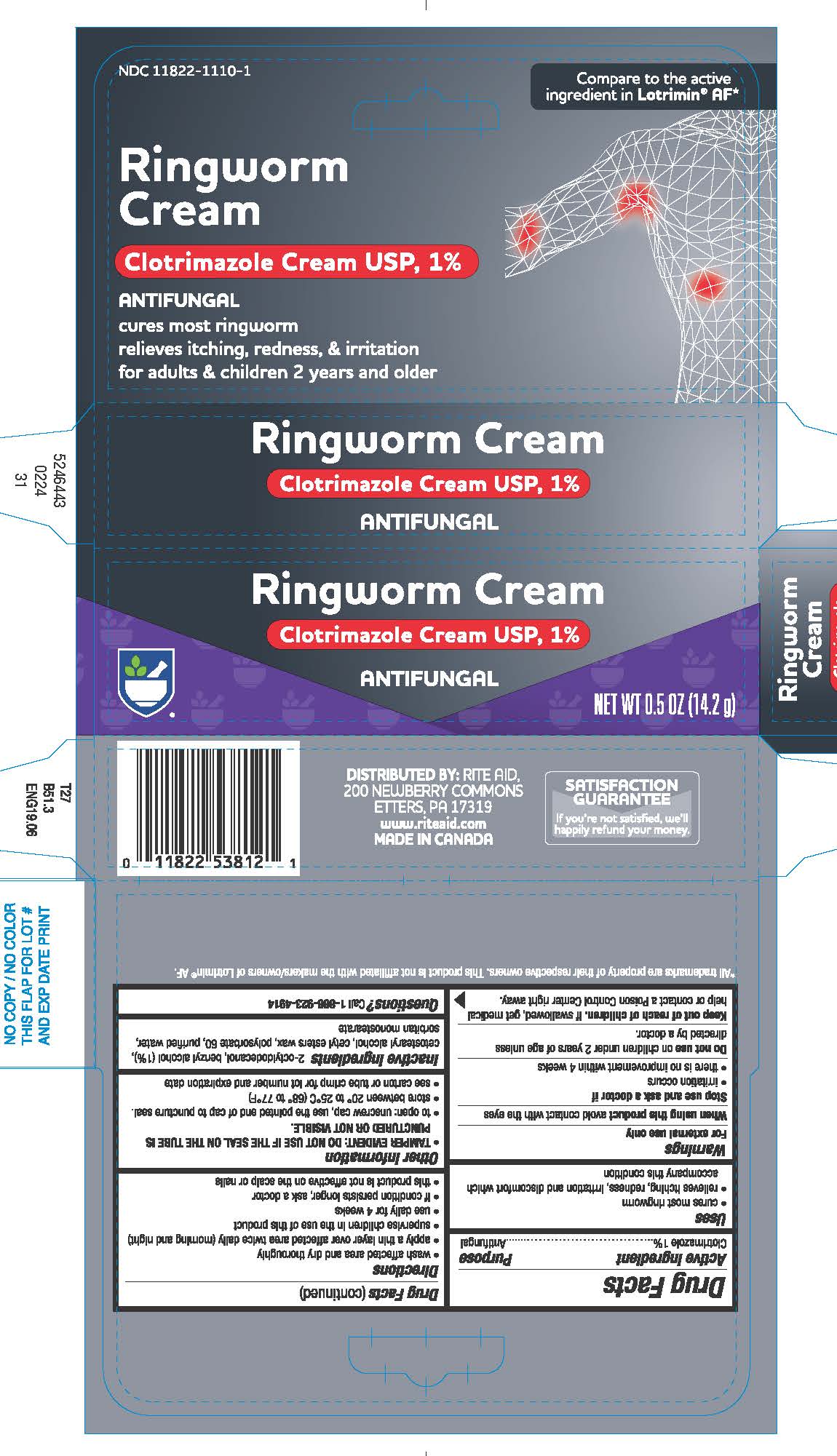
Active ingredient
Clotrimazole 1%
Uses
- cures most ringworm
- relieves itching, redness, irritation and discomfort which accompany this condition
Warnings
For external use only
When using this productavoid contact with eyes
Stop use and ask a doctor if
- irritation occurs
- there is no improvement within 4 weeks
Do not useon children under 2 years of age unless directed by a doctor.
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- use daily for 4 weeks
- if condition persists longer, ask a doctor
- this product is not effective on the scalp or nails
Other information
- To open: unscrew cap, use the pointed end of cap to puncture seal.
- store between 20°- 25°C (68° - 77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
benzyl alcohol (1%), cetostearyl alcohol, cetyl esters wax, 2-octyldodecanol, polysorbate 60, purified water, sorbitan monostearate
Questions?
Call
1-866-923-4914
DISTRIBUTED BY:
RITE AID, 30 HUNTER LANE,
CAMP HILL, PA 17011
PRINCIPAL DISPLAY PANEL - 14.2 g Tube Carton
 RITE
RITE
AID
®
PHARMACY
Ringworm Cream
Clotrimazole Cream USP, 1%
ANTIFUNGAL
NET WT 0.5 OZ (14.2 g)
