Uses
- Helps prevent sunburn.
- Higher SPF gives more sunburn protection.
- Provides moderate protection against sunburn.
Directions
- Apply smootly every morning before sun exposure and as needed.
- Apply to the forehead, nose, chin and both cheeks. Blend with circular motion and spread softly.
- Moderate sun protection product.
Other information
- Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risk of skin aging, skin cancer, and other harmful effects of the sun.
Inactive ingredients
Aqua (water), cyclomethicone, cyclopentasiloxane, talc, cetyl peg/ppg-10/1 dimethicone, polyglyceryl-4 isostearate, pisum sativum (pea) extract), peg-12 dimethicone crosspolymer, hexyl laurate, isostearyl neopentanoate, disteardimonium hectorite, cyclohexasiloxane, rosa centifolia flower water, sodium chloride, mannitol, dimethicone, propylene carbonate, propylene glycol, phenoxyethanol, octyldodecanol, butylene glycol, dimethiconol, methylparaben, propylparaben, cetearyl dimethicone crosspolymer, tocopheryl acetate, microcristallina cera (microcrystalline wax), methicone, polymethylsilsesquioxane, petrolatum, phospholipids, bha, bht, cyclodextrin, butylparaben, ethylparaben, cholesterol, faex extract (yeast extract), isobutylparaben, candelilla cera (euphorbia cerifera (candelilla) wax), cera alba (beeswax), cetearyl alcohol, cetearyl glucoside, copernicia cerifera cera (copernicia cerifera (carnauba) wax), polyglyceryl-2 dipolyhydroxystearate, olea europaea oil unsaponifiables (olea europaea (olive) oil unsaponifiables), glycosphingolipids, disodium succinate. May contain: CI 77891 (titanium dioxide), CI 77492 (iron oxides), CI 77491 (iron oxides), CI 77499 (iron oxides).
PRINCIPAL DISPLAY PANEL - 30 ml Carton - Beige 1
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)
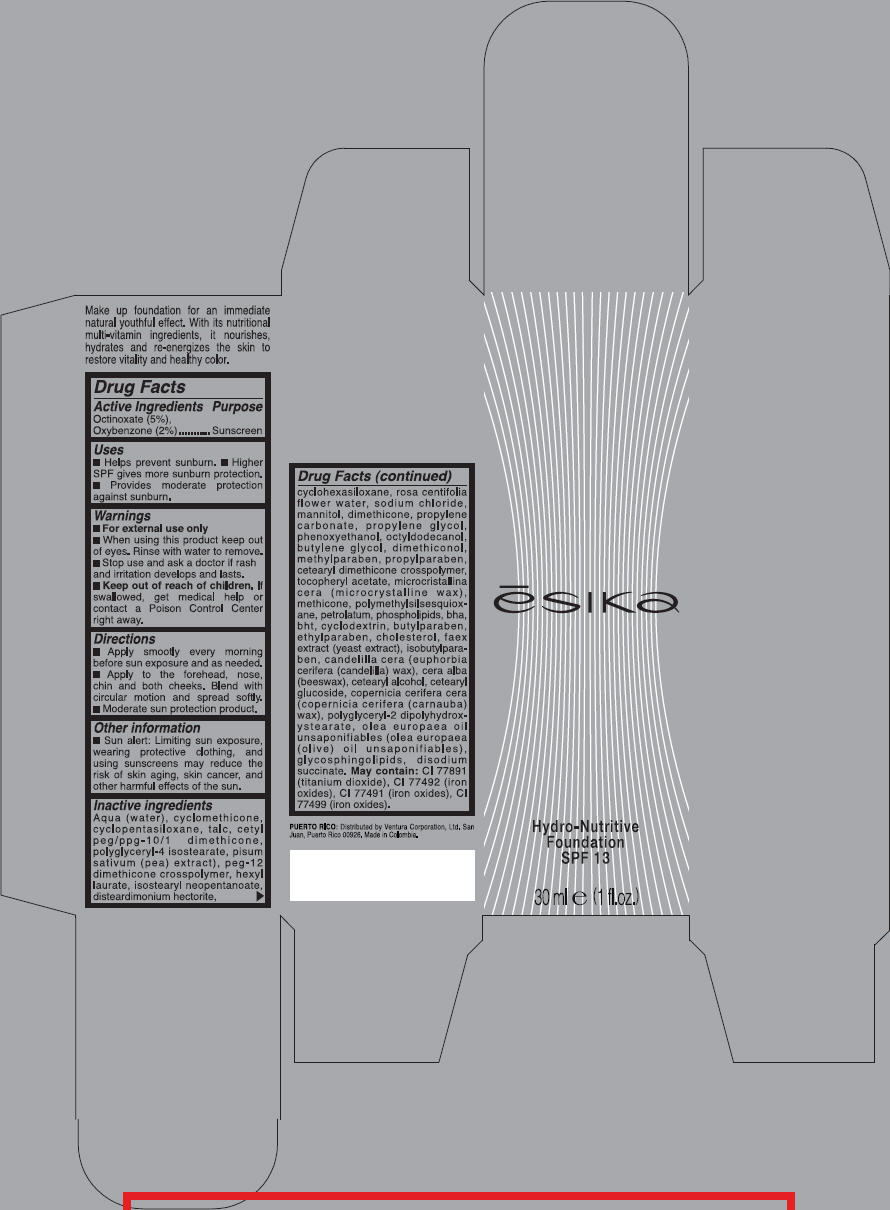
PRINCIPAL DISPLAY PANEL - 30 ml Carton - Beige 2
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)

PRINCIPAL DISPLAY PANEL - 30 ml Carton - Beige 3
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)

PRINCIPAL DISPLAY PANEL - 30 ml Carton - Beige 4
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)

PRINCIPAL DISPLAY PANEL - 30 ml Carton - Beige 5
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)

PRINCIPAL DISPLAY PANEL - 30 ml Carton - Beige 6
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)

PRINCIPAL DISPLAY PANEL - 30 ml Carton - Rosa 1
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)

PRINCIPAL DISPLAY PANEL - 30 ml Carton - Rosa 2
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)

PRINCIPAL DISPLAY PANEL - 30 ml Carton - Rosa 3
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)

PRINCIPAL DISPLAY PANEL - 30 ml Carton - Rosa 4
ēsika
Hydro-Nutritive
Foundation
SPF 13
30 ml e (1 fl.oz.)
