Uses
- For preparation of the skin prior to surgery
- Helps reduce bacteria that potentially can cause skin infections
Warnings
For external use only.
Do not use if you have a known sensitivity to iodine or any other ingredient in this product. Do not use in eyes. If product gets into eyes, flush immediately with water. Do not use on infants less than 2 months old due to the risk of increased blood iodine levels.
Directions
- Open package and remove bottle and swabs
- Unscrew cap by turning cap counter-clockwise
Skin Application:
- Apply to clean dry skin.
- Dip one swab into solution and stir vigorously for 10 seconds. Withdraw the swab slowly to avoid wiping solution off during removal.
- Scrub prep site for 2 minutes working from clean to dirty using both sides of the swab.
- Repeat steps 2 & 3 using second swab.
- Allow prep solution to dry. Do not blot.
Nasal Application:
- Use a tissue to clean the inside of both nostrils including the inside tip of nostril. Discard.
- Tilting the bottle slightly, dip one swab into solution and stir vigorously for 10 seconds. Withdraw the swab slowly to avoid wiping solution off during removal.

- Insert swab comfortably into one nostril and rotate for 15 seconds covering all surfaces. Then focus on the inside tip of nostril and rotate for an additional 15 seconds. (swab 1)

- Using a new swab: Repeat steps 2 & 3 with the other nostril. (swab 2)
- Repeat the application in both nostrils using a fresh swab each time. (swabs 3 & 4)

- Do not blow nose. If solution drips out of nose, it can be lightly dabbed with at tissue.
Inactive Ingredients:
ceteareth-25, lactic acid, lauramidopropylamine oxide, malic acid, polyquarternium-10, PPG-5-ceteth-10 phosphate, sodium hydroxide, sodium iodide, water, xylitol
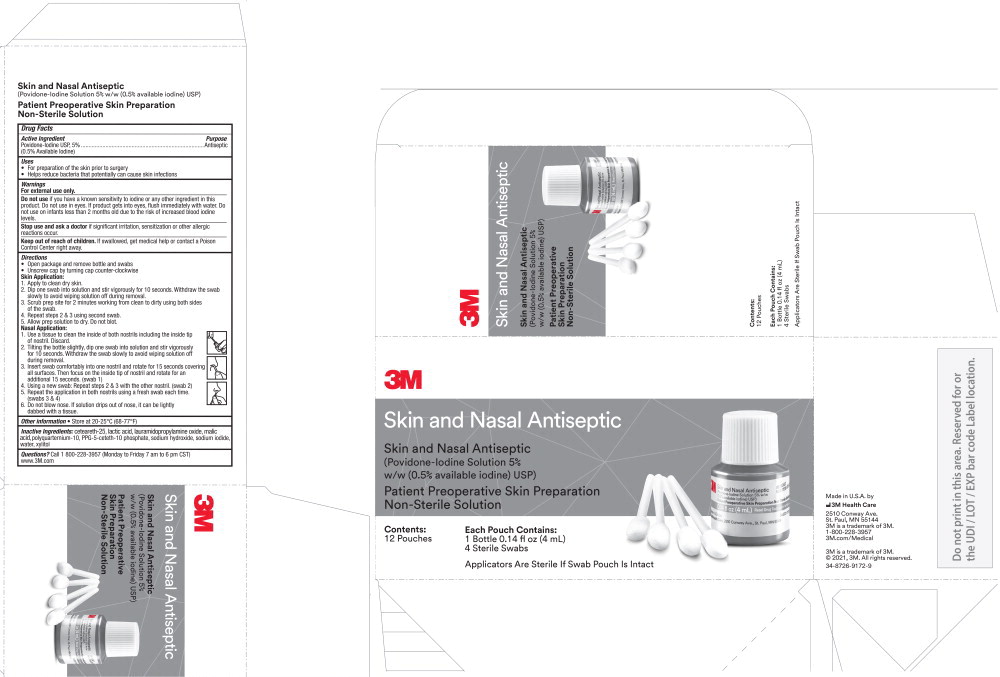
Principal Display Panel – Carton
3M Skin and Nasal Antiseptic
(Povidone-Iodine Solution 5% w/w (0.5% available iodine) USP)
Patient Preoperative Skin Preparation
Non-Sterile Solution
Contents:
12 pouches
Each Pouch Contains:
1 Bottle 0.14 fl oz (4 mL)
4 Sterile Swabs
Applicators Are Sterile If Swab Pouch Is Intact
Made in U.S.A. by
3M Health Care
2510 Conway Ave.
St. Paul, MN 55144
3M is a trademark of 3M
1-800-228-3957
3m.com/Medical
3M is a trademark of 3M
© 2021, 3M. All rights reserved.
34-8726-9172-9
Principal Display Panel – Pouch Label
NDC 17518-060-04
Not Made With Natural Rubber Latex
Do Not Reuse
3M Skin and Nasal Antiseptic
Skin and Nasal Antiseptic
(Povidone-Iodine Solution 5% w/w (0.5% available iodine) USP)
Patient Preoperative
Skin Preparation
Non-Sterile Solution
Each Pouch Contains:
1 Bottle 0.14 fl oz (4 mL)
4 Sterile Swabs
Applicators are sterile if swab pouch is intact
REF
192401
