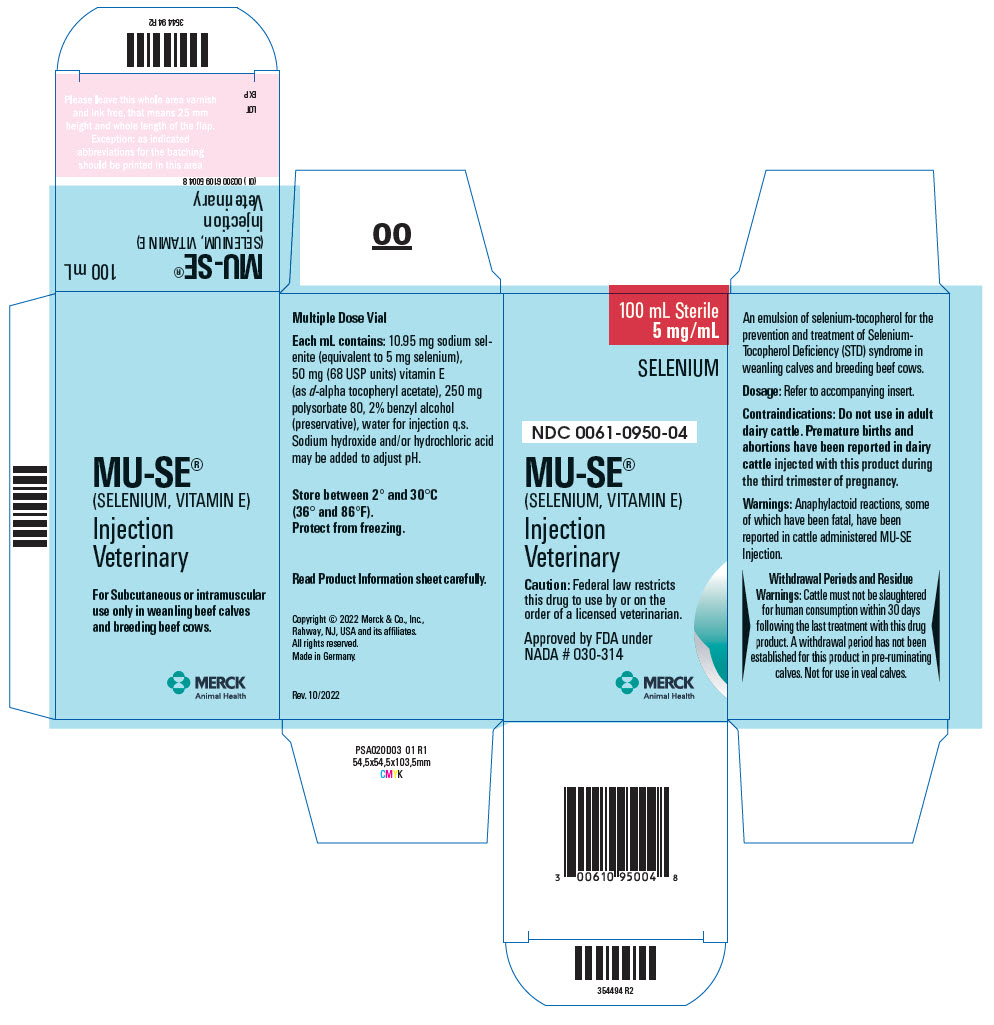
Approved by FDA under NADA # 030-314
PRODUCT INFORMATION
FOR VETERINARY USE ONLY
For Subcutaneous or intramuscular use only in weanling beef calves and breeding beef cows.
CAUTION Federal law restricts this drug to use by or on the order of a licensed veterinarian.
DESCRIPTION MU-SE (selenium, vitamin E) is an emulsion of selenium-tocopherol for the prevention and treatment of Selenium-Tocopherol Deficiency (STD) syndrome in weanling calves and breeding beef cattle. Each mL contains: 10.95 mg sodium selenite (equivalent to 5 mg selenium), 50 mg (68 USP units) vitamin E (as d-alpha tocopheryl acetate), 250 mg polysorbate 80, 2% benzyl alcohol (preservative), water for injection q.s. Sodium hydroxide and/or hydrochloric acid may be added to adjust pH.
ACTIONS It has been demonstrated that selenium and tocopherol exert physiological effects and that these effects are intertwined with sulfur metabolism. Additionally, tocopherol appears to have a significant role in the oxidation process, thus suggesting an interrelationship between selenium and tocopherol in overcoming sulfur-induced depletion and restoring normal metabolism. Although oral ingestion of adequate amounts of selenium and tocopherol would seemingly restore normal metabolism, it is apparent that the presence of sulfur and, perhaps, other factors interfere during the digestive process with proper utilization of selenium and tocopherol. When selenium and tocopherol are injected, they bypass the digestive process and exert their full metabolic effects promptly on cell metabolism. Anti-inflammatory action has been demonstrated by selenium-tocopherol in the Selye Pouch Technique and experimentally induced polyarthritis study in rats.
INDICATIONS MU-SE (selenium, vitamin E) is recommended for the prevention and treatment of STD syndrome in weanling calves and breeding beef cattle. Clinical signs are: stiffness and lameness; chronic, persistent diarrhea; unthriftiness; abortions and/or weak premature calves.
CONTRAINDICATION Do not use in adult dairy cattle. Premature births and abortions have been reported in dairy cattle injected with this product during the third trimester of pregnancy.
WARNINGS Anaphylactoid reactions, some of which have been fatal, have been reported in cattle administered the MU-SE product. Signs include excitement, sweating, trembling, ataxia, respiratory distress, and cardiac dysfunction.
DOSAGE AND ADMINISTRATION Inject subcutaneously or intramuscularly. Weanling calves: 1 mL per 200 pounds of body weight. Breeding beef cows: 1 mL per 200 pounds of body weight during the middle third of pregnancy, and 30 days before calving.
CAUTION Selenium is toxic if administered in excess. A fixed dose schedule is therefore important (read package insert for each selenium-tocopherol product carefully before using).
PRECAUTIONS Selenium-Tocopherol Deficiency (STD) syndrome produces a variety and complexity of symptoms often interfering with a proper diagnosis. Even in selenium deficient areas there are other disease conditions which produce similar clinical signs. It is imperative that all these conditions be carefully considered prior to treatment of STD syndrome. Serum selenium levels, elevated SGOT, and creatine levels may serve as aids in arriving at a diagnosis of STD, when associated with other indices.
Important Use only the selenium-tocopherol product recommended for each species. Each formulation is designed for the species indicated to produce the maximum efficacy and safety.