FULL PRESCRIBING INFORMATION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
nmCRPC and mHSPC
The recommended dose of NUBEQA is 600 mg (two 300 mg tablets) taken orally, twice daily, with food [see Clinical Pharmacology (12.3)].
Continue treatment until disease progression or unacceptable toxicity occurs.
Patients receiving NUBEQA should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or have had a bilateral orchiectomy.
Advise patients to swallow tablets whole with food, to take any missed dose as soon as they remember prior to the next scheduled dose, and not to take two doses together to make up for a missed dose.
mHSPC
For patients with mHSPC treated with NUBEQA in combination with docetaxel, administer the first of 6 cycles of docetaxel within 6 weeks after the start of NUBEQA treatment. Refer to docetaxel prescribing information for additional dosing information, including dosage modifications.
Treatment with NUBEQA may be continued until disease progression or unacceptable toxicity, even if a cycle of docetaxel is delayed, interrupted, or discontinued [see Dosage and Administration (2.2)].
2.2 Dosage Modification
If a patient experiences a greater than or equal to Grade 3 or an intolerable adverse reaction, withhold NUBEQA or reduce dosage to 300 mg twice daily until symptoms improve. NUBEQA may be resumed at a dose of 600 mg twice daily, when adverse reaction returns to baseline [see Adverse Reactions (6.1]). Dosage reduction below 300 mg twice daily is not recommended.
For patients who experience ischemic heart disease or seizure, additional dose modifications may be required [see Warnings and Precautions (5.1 and 5.2)].
3 DOSAGE FORMS AND STRENGTHS
Tablets (300 mg): white to off-white oval film-coated tablets marked with “300” on one side and “Bayer” on the other.
5 WARNINGS AND PRECAUTIONS
5.1 Ischemic Heart Disease
Ischemic heart disease, including fatal cases, occurred in patients receiving NUBEQA.
In a randomized study of patients with nmCRPC (ARAMIS), ischemic heart disease occurred in 3.2% of patients receiving NUBEQA and 2.5% receiving placebo, including Grade 3-4 events in 1.7% and 0.4%, respectively. Ischemic events led to death in 0.3% of patients receiving NUBEQA and 0.2% receiving placebo.
In a randomized study of patients with mHSPC (ARASENS), ischemic heart disease occurred in 3.2% of patients receiving NUBEQA with docetaxel and 2% receiving placebo with docetaxel, including Grade 3-4 events in 1.3% and 1.1%, respectively. Ischemic events led to death in 0.3% of patients receiving NUBEQA with docetaxel and 0% receiving placebo with docetaxel.
Monitor for signs and symptoms of ischemic heart disease. Optimize management of cardiovascular risk factors, such as hypertension, diabetes, or dyslipidemia. Discontinue NUBEQA for Grade 3-4 ischemic heart disease.
5.2 Seizure
Seizure occurred in patients receiving NUBEQA.
In ARAMIS, Grade 1-2 seizure occurred in 0.2% of patients receiving NUBEQA and 0.2% receiving placebo. Seizure occurred 261 and 456 days after initiation of NUBEQA.
In ARASENS, seizure occurred in 0.6% of patients receiving NUBEQA with docetaxel, including one Grade 3 event, and 0.2% receiving placebo with docetaxel. Seizure occurred 38 to 340 days after initiation of NUBEQA.
It is unknown whether anti-epileptic medications will prevent seizures with NUBEQA. Advise patients of the risk of developing a seizure while receiving NUBEQA and of engaging in any activity where sudden loss of consciousness could cause harm to themselves or others. Consider discontinuation of NUBEQA in patients who develop a seizure during treatment.
5.3 Embryo-Fetal Toxicity
The safety and efficacy of NUBEQA have not been established in females. Based on its mechanism of action, NUBEQA can cause fetal harm and loss of pregnancy when administered to a pregnant female [see Clinical Pharmacology (12.1)].
Advise males with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose of NUBEQA [see Use in Specific Populations (8.1, 8.3)].
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Non-Metastatic Castration Resistant Prostate Cancer
The safety of NUBEQA was evaluated in ARAMIS, a randomized (2:1), double-blind, placebo-controlled, multi-center clinical study, that enrolled patients who had non-metastatic castration-resistant prostate cancer (nmCRPC) [see Clinical Studies (14)]. Patients received either NUBEQA at a dose of 600 mg, or a placebo, twice a day. All patients in the ARAMIS study received a concomitant gonadotropin-releasing hormone (GnRH) analog or had a bilateral orchiectomy. Among patients who received NUBEQA, the median duration of exposure was 14.8 months (range: 0 to 44.3 months).
Serious adverse reactions occurred in 25% of patients receiving NUBEQA and in 20% of patients receiving placebo. Serious adverse reactions in ≥1% of patients who received NUBEQA included urinary retention, pneumonia and hematuria. Fatal adverse reactions occurred in 3.9% of patients receiving NUBEQA and 3.2% of patients receiving placebo. Fatal adverse reactions in patients who received NUBEQA included death (0.4%), cardiac failure (0.3%), cardiac arrest (0.2%), general physical health deterioration (0.2%), and pulmonary embolism (0.2%).
Permanent discontinuation of NUBEQA due to adverse reactions occurred in 9% of patients receiving NUBEQA. The most common adverse reactions requiring permanent discontinuation in patients who received NUBEQA included cardiac failure (0.4%), and death (0.4%).
Dosage interruptions due to adverse reactions occurred in 13% of patients treated with NUBEQA. The most common adverse reactions requiring dosage interruption in patients who received NUBEQA included hypertension (0.6%), diarrhea (0.5%), and pneumonia (0.5%).
Dosage reductions due to adverse reactions occurred in 6% of patients treated with NUBEQA. The most common adverse reactions requiring dosage reduction in patients treated with NUBEQA included fatigue (0.7%), hypertension (0.3%), and nausea (0.3%).
The most common (>2% with a ≥2% increase compared to placebo) adverse reactions, including laboratory test abnormalities, were AST increased, neutrophil count decreased, fatigue, bilirubin increased, pain in extremity, and rash.
Table 1 summarizes the adverse reactions in ARAMIS.
|
Adverse Reaction |
NUBEQA (n=954) |
Placebo (n=554) |
||
|
All Grades |
Grades 3 or 4 % |
All Grades |
Grade 3 or 4 |
|
|
Fatigue* |
16 |
0.6 |
11 |
1.1 |
|
Pain in extremity |
6 |
0 |
3 |
0.2 |
|
Rash† |
4 |
0.1 |
1.4 |
0 |
Clinically relevant adverse reactions occurring in 2% or more of patients treated with NUBEQA included ischemic heart disease (4%) and heart failure (2.1%).
Table 2 summarizes the laboratory test abnormalities in ARAMIS.
| Laboratory Abnormality | NUBEQA (N=954)* | Placebo (N=554)* |
||
|---|---|---|---|---|
| All Grades†
% | Grade 3-4 % | All Grades % | Grade 3-4 % |
|
|
||||
|
AST increased |
23 |
0.5 |
14 |
0.2 |
|
Neutrophil count decreased |
20 |
4 |
9 |
0.6 |
|
Bilirubin increased |
16 |
0.1 |
7 |
0 |
Metastatic Hormone-Sensitive Prostate Cancer
The safety of NUBEQA, in combination with docetaxel, was evaluated in ARASENS, a randomized (1:1), double-blind, placebo-controlled, multi-center clinical study, that enrolled patients who had mHSPC [see Clinical Studies (14)]. Patients were to receive either NUBEQA at a dose of 600 mg, or a placebo, twice a day in combination with docetaxel at a dose of 75 mg/m2 every 21 days for 6 cycles. All patients in the ARASENS study received a concomitant gonadotropin-releasing hormone (GnRH) analog or had a bilateral orchiectomy. Patients with a medical history of seizure were allowed to enter the study. Among patients who received NUBEQA, the median duration of exposure was 41 months (range: 0.1 to 56.5 months) vs. 16.7 months (range 0.3 to 55.8) with placebo. Eighty-eight percent and 86% of patients received the 6 planned cycles of docetaxel, in the NUBEQA with docetaxel arm and placebo with docetaxel arm, respectively.
Serious adverse reactions occurred in 45% of patients receiving NUBEQA with docetaxel and in 42% of patients receiving placebo with docetaxel, respectively. Serious adverse reactions in ≥ 2% of patients who received NUBEQA with docetaxel included febrile neutropenia (6%), neutrophil count decreased (2.8%), musculoskeletal pain (2.6%) and pneumonia (2.6%). Fatal adverse reactions occurred in 4% of patients receiving NUBEQA with docetaxel and 4% of patients receiving placebo with docetaxel. Fatal adverse reactions in patients who received NUBEQA included COVID-19/COVID-19 pneumonia (0.8%), myocardial infarction (0.3%), and sudden death (0.3%).
Permanent discontinuation of NUBEQA due to adverse reactions occurred in 14% of patients treated in the NUBEQA with docetaxel arm. The most common adverse reactions which resulted in permanent discontinuation of NUBEQA were rash (1.1%), musculoskeletal pain (0.9%), and aspartate aminotransferase (AST) increased (0.9%).
Dosage interruptions of NUBEQA due to adverse reactions occurred in 23% of patients treated in the NUBEQA with docetaxel arm. The most common (>2%) adverse reactions requiring dosage interruption of NUBEQA were alanine aminotransferase (ALT) increased (3.2%), AST increased (3.1%) and febrile neutropenia (2.1%).
Dosage reductions of NUBEQA due to adverse reactions occurred in 9% of patients treated in the NUBEQA with docetaxel arm. The most common (>2%) adverse reactions requiring dosage reduction of NUBEQA were ALT increased (2.8%) and AST increased (2.5%).
The most common (>10% with a ≥2% increase over placebo with docetaxel) adverse reactions are constipation, rash decreased appetite, hemorrhage, weight increased, and hypertension. The most common laboratory test abnormalities (≥30%) are anemia, hyperglycemia, lymphocyte count decreased, neutrophil count decreased, AST increased, ALT increased, and hypocalcemia.
Table 3 summarizes the adverse reactions in ARASENS.
|
||||
|
Adverse Reaction |
NUBEQA with docetaxel (n=652) |
Placebo with docetaxel (n=650) |
||
|
All Grades |
Grades 3 or 4 |
All Grades |
Grades 3 or 4 |
|
|
23 |
0.3 |
20 |
0.3 |
|
20 |
1.8 |
15 |
0.2 |
|
19 |
0.2 |
13 |
0.6 |
|
18 |
1.4 |
13 |
1.4 |
|
18 |
2.1 |
16 |
1.2 |
|
14 |
7 |
10 |
3.6 |
Hemorrhage includes hematuria, epistaxis, anal hemorrhage, hemorrhoidal hemorrhage, rectal hemorrhage, upper gastrointestinal hemorrhage, hemoptysis, hemorrhage urinary tract, hemorrhagic stroke, subarachnoid hemorrhage, lower gastrointestinal hemorrhage, cystitis hemorrhagic, gastrointestinal hemorrhage, hemorrhage subcutaneous, intra-abdominal hemorrhage, nail bed bleeding, subdural hemorrhage
Clinically relevant adverse reactions in < 10% of patients who received NUBEQA with docetaxel included fractures (8%), ischemic heart disease (3.2%), seizures (0.6%), and drug-induced liver injury (0.3%).
Table 4 summarizes laboratory test abnormalities in the ARASENS study.
|
||||
|
Laboratory Abnormality |
NUBEQA with docetaxel† (N=652) |
Placebo with docetaxel† (N=650) |
||
|
All Grades % |
Grade 3-4 % |
All Grades % |
Grade 3-4 % |
|
|
72 |
6 |
71 |
7 |
|
57 |
7 |
53 |
10 |
|
52 |
12 |
49 |
13 |
|
49 |
33 |
44 |
31 |
|
40 |
3.6 |
35 |
2.3 |
|
37 |
3.7 |
31 |
2.9 |
|
31 |
2.8 |
28 |
1.9 |
Clinically relevant laboratory test abnormalities in < 30% of patients who received NUBEQA with docetaxel included blood bilirubin increased (all grades 20%, Grade 3-4 0.5%) compared to placebo with docetaxel (all grades 10%, grades 3-4 0.3%).
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on NUBEQA
Combined P-gp and Strong or Moderate CYP3A4 Inducer
Concomitant use of NUBEQA with a combined P-gp and strong or moderate CYP3A4 inducer decreases darolutamide exposure which may decrease NUBEQA activity [see Clinical Pharmacology (12.3)]. Avoid concomitant use of NUBEQA with combined P-gp and strong or moderate CYP3A4 inducers.
Combined P-gp and Strong CYP3A4 Inhibitors
Concomitant use of NUBEQA with a combined P-gp and strong CYP3A4 inhibitor increases darolutamide exposure [see Clinical Pharmacology (12.3)] which may increase the risk of NUBEQA adverse reactions. Monitor patients more frequently for NUBEQA adverse reactions and modify NUBEQA dosage as needed [see Dosage and Administration (2.2)].
7.2 Effects of NUBEQA on Other Drugs
Breast Cancer Resistance Protein (BCRP) and Organic Anion Transporting Polypeptides (OATP) 1B1 and 1B3 Substrates
NUBEQA is an inhibitor of BCRP transporter. Concomitant use of NUBEQA increases the AUC and Cmax of BCRP substrates [see Clinical Pharmacology (12.3)], which may increase the risk of BCRP substrate-related toxicities.
Avoid concomitant use with drugs that are BCRP substrates where possible. If used together, monitor patients more frequently for adverse reactions, and consider dose reduction of the BCRP substrate drug.
NUBEQA is an inhibitor of OATP1B1 and OATP1B3 transporters. Concomitant use of NUBEQA may increase the plasma concentrations of OATP1B1 or OATP1B3 substrates. Monitor patients more frequently for adverse reactions of these drugs and consider dose reduction while patients are taking NUBEQA [see Clinical Pharmacology (12.3)],
Review the prescribing information of the BCRP, OATP1B1 and OATP1B3 substrates when used concomitantly with NUBEQA.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The safety and efficacy of NUBEQA have not been established in females. Based on its mechanism of action, NUBEQA can cause fetal harm and loss of pregnancy [see Clinical Pharmacology (12.1)]. Animal embryo-fetal developmental toxicology studies were not conducted with darolutamide. There are no human data on the use of NUBEQA in pregnant females.
8.3 Females and Males of Reproductive Potential
Contraception
Males
Based on the mechanism of action, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose of NUBEQA [see Use in Specific Populations (8.1)].
Infertility
Males
Based on animal studies, NUBEQA may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of NUBEQA in pediatric patients have not been established.
8.5 Geriatric Use
Of the 954 patients who received NUBEQA in ARAMIS, 88% of patients were 65 years and over, and 49% were 75 years and over. Of the 652 patients who received NUBEQA in ARASENS, 63% of patients were 65 years and over, and 16% were 75 years and over. No overall differences in safety or efficacy were observed between these patients and younger patients in both studies.
8.6 Renal Impairment
Patients with severe renal impairment (eGFR 15–29 mL/min/1.73 m2) who are not receiving hemodialysis have a higher exposure to NUBEQA and reduction of the dose is recommended [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]. No dose reduction is needed for patients with mild or moderate renal impairment (eGFR 30-89 mL/min/1.73 m2). The effect of end stage renal disease (eGFR ≤15 mL/min/1.73 m2) on darolutamide pharmacokinetics is unknown.
8.7 Hepatic Impairment
Patients with moderate hepatic impairment (Child-Pugh Class B) have a higher exposure to NUBEQA and reduction of the dose is recommended [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)]. No dose reduction is needed for patients with mild hepatic impairment. The effect of severe hepatic impairment (Child-Pugh C) on darolutamide pharmacokinetics is unknown.
10 OVERDOSAGE
There is no known specific antidote for darolutamide overdose. The highest dose of NUBEQA studied clinically was 900 mg twice daily, equivalent to a total daily dose of 1800 mg. No dose limiting toxicities were observed with this dose.
Considering the saturable absorption and the absence of evidence for acute toxicity, an intake of a higher than recommended dose of darolutamide is not expected to lead to systemic toxicity in patients with intact hepatic and renal function [see Clinical Pharmacology (12.3)].
In the event of intake of a higher than recommended dose in patients with severe renal impairment or moderate hepatic impairment, if there is suspicion of toxicity, interrupt NUBEQA treatment and undertake general supportive measures until clinical toxicity has been diminished or resolved. If there is no suspicion of toxicity, NUBEQA treatment can be continued with the next dose as scheduled.
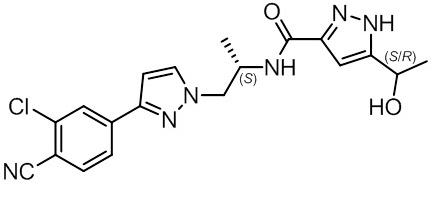
11 DESCRIPTION
NUBEQA is an androgen receptor inhibitor. The chemical name is N-{(2S)-1-[3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl]propan-2-yl}-5-(1-hydroxyethyl)-1H-pyrazole-3-carboxamide.
- The molecular weight is 398.85 and the molecular formula is C19H19Cl N6O2. The structural formula is:
Darolutamide is an optically active with a specific rotation value [α]20D= 72.2 o*mL/(dm*g), white to greyish- or yellowish white crystalline powder, that is soluble in tetrahydrofuran, but practically insoluble in aqueous medium. Darolutamide has a pKa of 11.75.
NUBEQA (darolutamide) is supplied as film-coated tablets containing 300 mg of darolutamide for oral use. The inactive ingredients of the tablet are: calcium hydrogen phosphate, croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone K 30, hypromellose 15 cP, macrogol 3350, and titanium dioxide.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Darolutamide is an androgen receptor (AR) inhibitor. Darolutamide competitively inhibits androgen binding, AR nuclear translocation, and AR-mediated transcription. A major metabolite, ketodarolutamide, exhibited similar in vitro activity to darolutamide. In addition, darolutamide functioned as a progesterone receptor (PR) antagonist in vitro (approximately 1% activity compared to AR). Darolutamide decreased prostate cancer cell proliferation in vitro and tumor volume in mouse xenograft models of prostate cancer.
12.2 Pharmacodynamics
PSA reduction was observed in CRPC patients receiving darolutamide doses of 100-900 mg twice a day, reaching a plateau of PSA reduction at the 600 mg twice daily dose.
Twice daily dosing of 600 mg darolutamide in nmCRPC patients resulted in undetectable PSA levels in 24.2% of patients at 12 months compared to 0.4% of patients in the placebo arm.
Twice daily dosing of 600 mg darolutamide in combination with docetaxel in mHSPC patients resulted in undetectable PSA levels in 60.2% of patients at 12 months compared to 26.1% of patients in the placebo with docetaxel arm.
12.3 Pharmacokinetics
Following administration of 600 mg twice daily, darolutamide mean (%CV) steady-state peak plasma concentration (Cmax) is 4.79 mg/L (30.9%) and area under the plasma concentration-time curve from time 0 to 12 hours (AUC12h) is 52.82 h•µg/mL (33.9%). Steady-state is reached 2–5 days after repeated dosing with food, with an approximate 2-fold accumulation.
The exposure (Cmax and AUC12) of the darolutamide and the active metabolite ketodarolutamide increase in a nearly dose-proportional manner in the dose range of 100 to 700 mg (0.17 to 1.17 times the approved recommended dosage). No further increase in darolutamide exposure was observed at 900 mg twice daily (1.5 times the approved recommended dosage).
Absorption
Darolutamide Cmax is reached approximately 4 hours after administration of a single 600 mg oral dose.
The absolute bioavailability is approximately 30% following oral administration of a NUBEQA tablet containing 300 mg darolutamide under fasted conditions.
Food Effect
Bioavailability of darolutamide increased by 2.0- to 2.5‑fold when administered with food. A similar increase of exposure was observed for the active metabolite ketodarolutamide.
Distribution
The apparent volume of distribution of darolutamide after intravenous administration is 119 L.
Protein binding is 92% for darolutamide and 99.8% for the active metabolite, ketodarolutamide. Serum albumin is the main binding protein for darolutamide and ketodarolutamide.
Elimination
The effective half-life of darolutamide and ketodarolutamide is approximately 20 hours in patients. The clearance (%CV) of darolutamide following intravenous administration is 116 mL/min (39.7%).
Metabolism
Darolutamide is primarily metabolized by CYP3A4, as well as by UGT1A9 and UGT1A1. Ketodarolutamide total exposure in plasma is 1.7‑fold higher compared to darolutamide.
Excretion
After a single radiolabeled dose as an oral solution, a total of 63.4% of darolutamide‑related material is excreted in the urine (approximately 7% unchanged) and 32.4% (approximately 30% unchanged) in the feces. More than 95% of the dose was recovered within 7 days after administration.
Specific Populations
No clinically significant differences in the pharmacokinetics of darolutamide were observed based on age (41-95 years), race (White, Asian, Black or African American), mild to moderate renal impairment (eGFR 30–89 mL/min/1.73m2), or mild hepatic impairment.
In non-cancer subjects with severe renal impairment (eGFR 15–29 mL/min/1.73 m2) not receiving dialysis or with moderate hepatic impairment (Child-Pugh Class B), NUBEQA exposure increased by about 2.5- and 1.9-fold, respectively, compared to healthy subjects.
The effect of end-stage renal disease (eGFR <15 mL/min/1.73 m2) or severe hepatic impairment (Child-Pugh C) on darolutamide pharmacokinetics has not been studied.
Combined P-gp and Strong CYP3A4 Inducers
Concomitant use of rifampicin (a combined P-gp and strong CYP3A4 inducer) decreased mean darolutamide AUC0-72 by 72% and Cmax by 52%. The decrease of darolutamide exposure by moderate CYP3A4 inducers is expected to be in the range of 36% – 58%.
Combined P-gp and Strong CYP3A4 Inhibitors
Itraconazole (a strong combined CYP3A4 and P-gp inhibitor) increased mean darolutamide AUC0-72 by 1.7- and Cmax by 1.4-fold.
CYP3A4 substrates
Concomitant use of darolutamide decreased the mean AUC and Cmax of midazolam (CYP3A4 substrate) by 29% and 32%, respectively. No clinically relevant differences in the pharmacokinetics of midazolam were observed when used concomitantly with darolutamide.
BCRP, OATP1B1 and OATP1B3 Substrates
Concomitant use of darolutamide increased the mean AUC and Cmax of rosuvastatin (BCRP, OATP1B1 and OATP1B3 substrate) by approximately 5-fold.
Docetaxel
Administration of darolutamide in combination with docetaxel resulted in no clinically relevant changes in the pharmacokinetics of docetaxel in mHSPC patients. There were no clinically relevant changes in the pharmacokinetics of darolutamide, when used in combination with docetaxel.
P-gp Substrates
No clinically relevant differences in the pharmacokinetics of dabigatran (P-gp substrate) were observed when used concomitantly with darolutamide.
In vitro, darolutamide did not inhibit the major CYP enzymes (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4) or transporters (MRP2, BSEP, OATs, OCTs, MATEs, OATP2B1, and NTCP) at clinically relevant concentrations.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of darolutamide have not been conducted.
Darolutamide was clastogenic in an in vitro chromosome aberration assay in human peripheral blood lymphocytes. Darolutamide did not induce mutations in the bacterial reverse mutation (Ames) assay and was not genotoxic in the in vivo combined bone marrow micronucleus assay and the Comet assay in the liver and duodenum of the rat.
Fertility studies in animals have not been conducted with darolutamide. In repeat-dose toxicity studies in male rats (up to 26 weeks) and dogs (up to 39 weeks), tubular dilatation of testes, hypospermia, and atrophy of seminal vesicles, testes, prostate gland and epididymides were observed at doses ≥ 100 mg/kg/day in rats (0.6 times the human exposure based on AUC) and ≥ 50 mg/kg/day in dogs (approximately 1 times the human exposure based on AUC).
14 CLINICAL STUDIES
Non-Metastatic Castration Resistant Prostate Cancer
ARAMIS (NCT02200614) was a multicenter, double-blind, placebo-controlled clinical trial in 1509 patients with non-metastatic castration resistant prostate cancer with a prostate-specific antigen doubling time (PSADT) of ≤ 10 months. Randomization was stratified by PSADT and use of bone-targeted therapy at study entry. Patients with pelvic lymph nodes less than 2 cm in short axis below the aortic bifurcation and patients with a history of seizures were eligible to enter the study. Absence or presence of metastasis was assessed by blinded independent central review (BICR). PSA results were not blinded and were not used for treatment discontinuation.
Patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n=955) or matching placebo (n=554). Treatment continued until radiographic disease progression as assessed by CT, MRI, 99mTc bone scan by BICR, unacceptable toxicity or withdrawal. All patients received a gonadotropin-releasing hormone (GnRH) analog concurrently or had a bilateral orchiectomy.
The following patient demographics and disease characteristics were balanced between treatment arms. The median age was 74 years (range 48–95) and 9% of patients were 85 years of age or older. The racial distribution included 79% White, 13% Asian, 3% Black or African American, while ethnicity included 3.1% Hispanic or Latino patients. A majority of patients (73%) had a Gleason score of 7 or higher at diagnosis. The median PSADT was 4.5 months. Forty-two percent of patients in both treatment arms had prior surgery or radiotherapy to the prostate. Eleven percent of patients had enlarged pelvic lymph nodes less than 2 cm at study entry. Six percent of patients were retrospectively identified by BICR as having metastases at baseline. Seventy-three percent of patients received prior treatment with an anti-androgen (bicalutamide or flutamide). All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 at study entry. There were 12 patients enrolled on the NUBEQA arm with a history of seizure. At baseline, 47% of patients reported no pain on the Brief Pain Inventory-Short Form (a 7-day diary average of the daily worst pain item).
The major efficacy endpoint was metastasis free survival (MFS), defined as the time from randomization to the time of first evidence of BICR-confirmed distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first. Distant metastasis was defined as new bone or soft tissue lesions or enlarged lymph nodes above the aortic bifurcation. Overall survival (OS), time to pain progression, and time to initiation of cytotoxic chemotherapy, were additional efficacy endpoints.
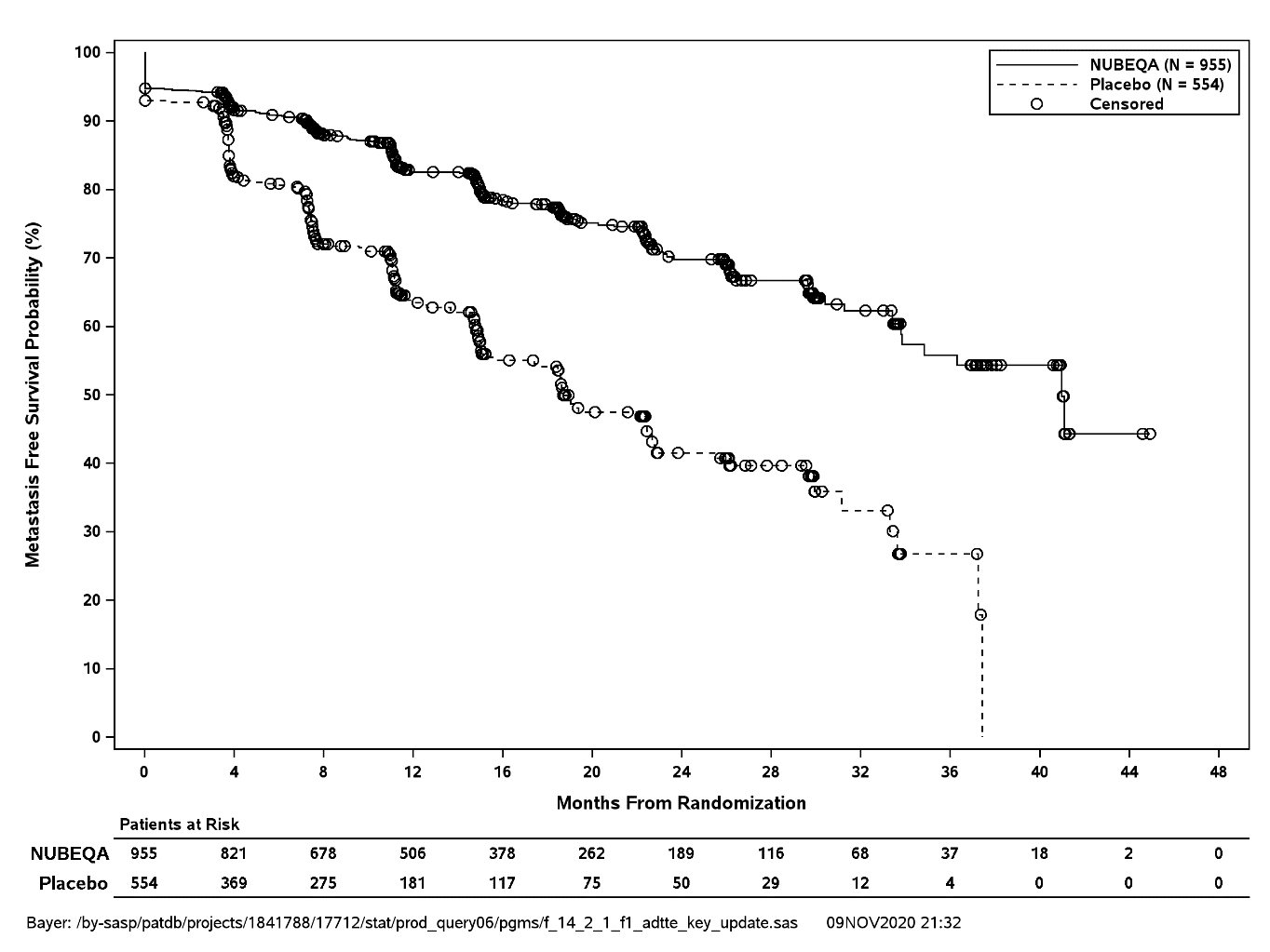
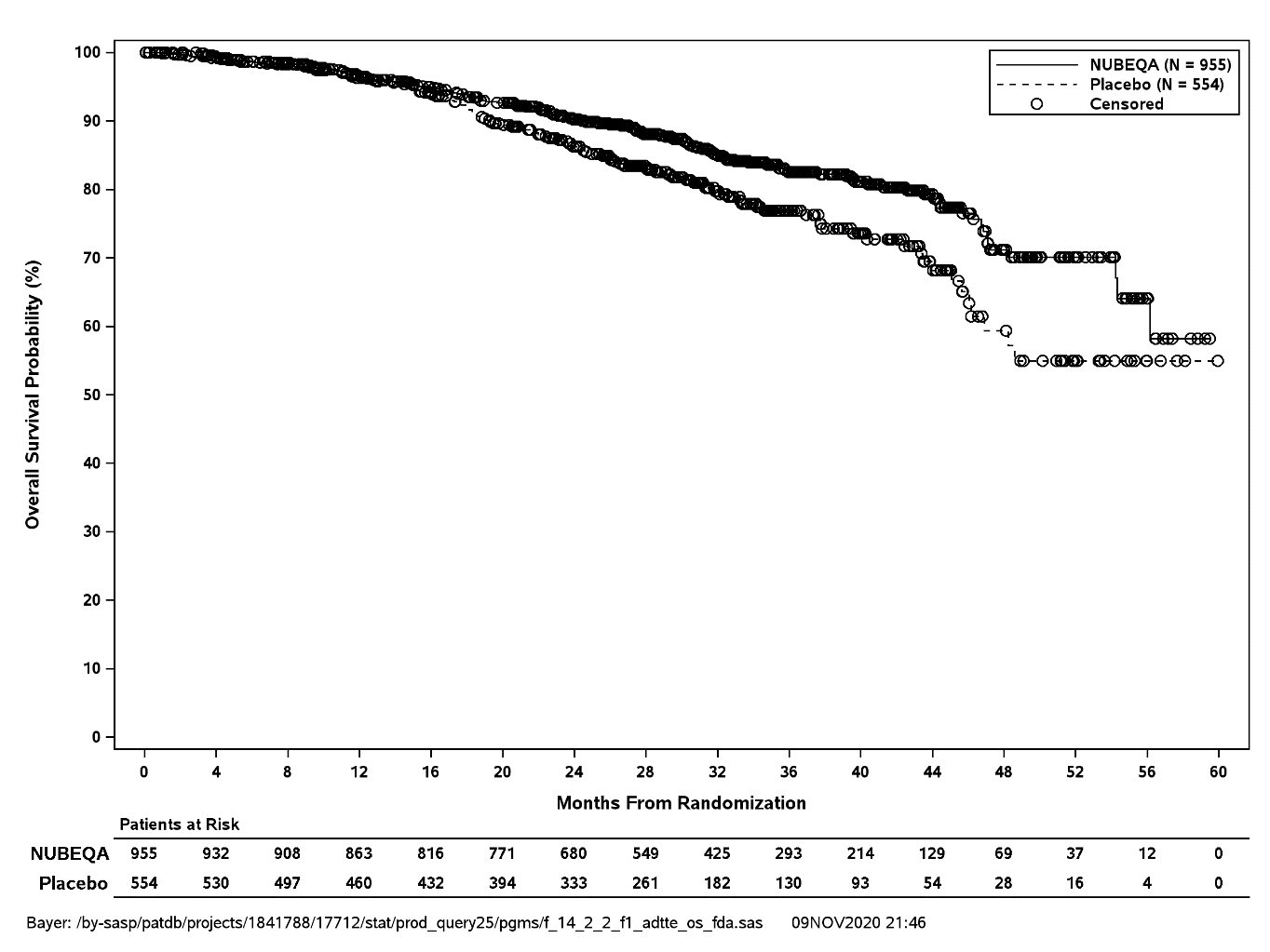
Treatment with NUBEQA resulted in a statistically significant improvement in MFS compared to placebo. At the protocol-specified final analysis of OS, treatment with NUBEQA resulted in a statistically significant improvement in OS compared to placebo. The final analysis of OS and time to initiation of cytotoxic chemotherapy was event-driven and conducted after 254 OS events had occurred. The efficacy results from ARAMIS are summarized in Table 5 and Figure 1.
|
||
|
NUBEQA (N=955) |
Placebo (N=554) |
|
|
Metastasis-free survival* |
||
|
Distant Metastasis or Death (%) |
221 (23) |
216 (39) |
|
Median, months (95% CI)† |
40.4 (34.3, NR) |
18.4 (15.5, 22.3) |
|
Hazard Ratio (95% CI)‡ |
0.41 (0.34, 0.50) |
|
|
P-value |
<0.0001 |
|
|
Overall survival§ |
||
|
Deaths (%) |
148 (15) |
106 (19) |
|
Median, months (95% CI) |
NR (56.1, NR) |
NR (46.9, NR) |
|
Hazard Ratio (95% CI)‡ |
0.69 (0.53, 0.88) |
|
|
P-value¶ |
0.003 |
|
|
NR: not reached | ||
MFS results were consistent across patient subgroups for PSADT (≤ 6 months or > 6 months) or prior use of bone-targeting agents (yes or no).
Treatment with NUBEQA resulted in a statistically significant delay in time to pain progression (HR = 0.65, 95% CI= 0.53, 0.79; p < 0.0001). Time to pain progression was defined as at least a 2-point worsening from baseline of the pain score on Brief Pain Inventory Short Form or initiation of opioids and reported in 28% of all patients on study.
Treatment with NUBEQA resulted in a statistically significant delay in the initiation of cytotoxic chemotherapy (HR = 0.58, 95% CI = 0.44, 0.76; p < 0.0001).
Metastatic Hormone-Sensitive Prostate Cancer
ARASENS (NCT02799602) was a multicenter, double-blind, placebo-controlled clinical trial in 1306 patients with mHSPC. Patients were randomized 1:1 to receive 600 mg darolutamide orally twice daily (n=651) or matching placebo (n=655), concomitantly with 75 mg/m2 of docetaxel for 6 cycles. All patients received a gonadotropin-releasing hormone (GnRH) analog concurrently or had a bilateral orchiectomy. Treatment with NUBEQA or placebo continued until symptomatic progressive disease, change of antineoplastic therapy, or unacceptable toxicity. Patients with regional lymph node involvement only (M0) were excluded from the study. Patients were stratified by extent of disease (non–regional lymph nodes metastases only (M1a), bone metastases with or without lymph node metastases (M1b) or visceral metastases with or without lymph node metastases or with or without bone metastases (M1c)) and by alkaline phosphatase level (< or ≥ upper limit of normal) at study entry.
The following patient demographics and disease characteristics were balanced between treatment arms. The median age was 67 years (range 41–89) and 17% of patients were 75 years of age or older. The racial distribution included 52% White, 36% Asian, 4% Black, while ethnicity included 7% Hispanic patients. Seventy-eight percent (78%) of patients had a Gleason score of ≥8 at diagnosis. Seventy one percent (71%) of patients had an ECOG performance status score of 0 and 29% patients had an ECOG performance status score of 1. There were 86% of patients with de novo and 13% with recurrent disease. At initial diagnosis of metastatic disease, 3% had M1a, 83% had M1b and 14% had M1c disease; alkaline phosphatase was < ULN in 45% of patients and ≥ ULN in 55% of patients; median PSA level at baseline was 30 µg/L and 24 µg/L for NUBEQA vs placebo group, respectively. Patients with a medical history of seizure were allowed to enter the study, and 4 patients (0.6%) were enrolled in the NUBEQA with docetaxel arm.
The major efficacy outcome measure was overall survival (OS). Time to pain progression was an additional efficacy outcome measure.
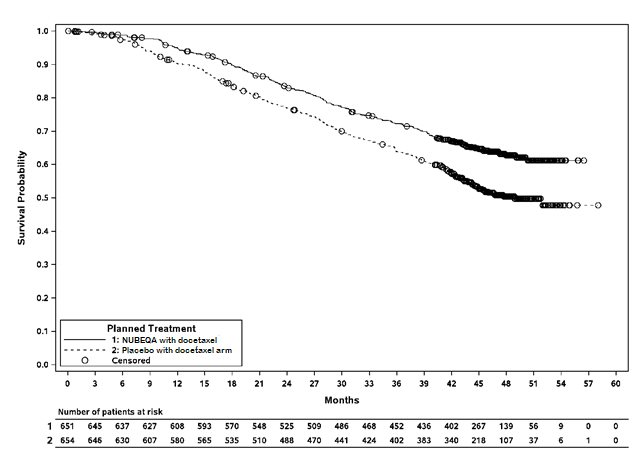
Treatment with NUBEQA with docetaxel resulted in a statistically significant improvement in OS compared to placebo with docetaxel. OS results were consistent across stratified subgroups (extent of disease and alkaline phosphatase level).
|
||
|
NUBEQA with docetaxel |
Placebo with docetaxel |
|
|
Overall survival |
||
|
229 (35) |
304 (46) |
|
NR (NR, NR) |
48.9 (44.4, NR) |
|
0.68 (0.57, 0.80) |
|
|
<0.0001 |
|
NR: not reached
Treatment with NUBEQA with docetaxel resulted in a statistically significant delay in time to pain progression (HR = 0.79, 95% CI= 0.66, 0.95; 1-sided p value = 0.006). Time to pain progression was defined as the time from randomization to the time of pain progression. Pain progression is defined as:
- •
- An increase of 2 or more points in the “worst pain in 24 hours” score (WPS) from nadir observed at 2 consecutive evaluations at least 4 weeks apart, or initiation of short- or long-acting opioid use for pain for at least 7 consecutive days, for asymptomatic patients (WPS=0) at baseline
- •
- An increase of 2 or more points in the “worst pain in 24 hours” score (WPS) from nadir observed at 2 consecutive evaluations at least 4 weeks apart, and a WPS of 4 or greater, or initiation of short- or long-acting opioid use for pain for at least 7 consecutive days, for symptomatic patients (WPS ≥ 1) at baseline
Patients with opioid use within 4 weeks before randomization were censored at randomization in this analysis (125 patients (19%) in the NUBEQA with docetaxel arm and 118 patients (18%) in the placebo with docetaxel arm).
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
NUBEQA (darolutamide) 300 mg film-coated tablets are white to off-white, oval shaped tablets, marked with “300” on one side, and “BAYER” on the other side. NUBEQA 300 mg tablets are available in bottles of 120 tablets.
NDC 50419-395-01
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Keep the bottle tightly closed after first opening.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information)
Ischemic Heart Disease
Inform patients that NUBEQA has been associated with an increased risk of ischemic heart disease. Advise patients to seek immediate medical attention if any symptoms suggestive of an ischemic heart disease event occur [see Warnings and Precautions (5.1)].
Seizure
Inform patients that NUBEQA has been associated with an increased risk of seizure. Discuss conditions that may predispose to seizures and medications that may lower the seizure threshold. Advise patients of the risk of engaging in any activity where sudden loss of consciousness could cause serious harm to themselves or others. Inform patients to contact their healthcare provider right away if they have loss of consciousness or seizure [see Warnings and Precautions (5.2)].
Embryo-Fetal Toxicity
Inform patients that NUBEQA can be harmful to a developing fetus and can cause loss of pregnancy [see Use in Specific Populations (8.1)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose of NUBEQA [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
Dosage and Administration
Inform patients receiving concomitant gonadotropin-releasing hormone (GnRH) analog therapy that they need to maintain this treatment during the course of treatment with NUBEQA.
Instruct patients to take their dose of two tablets (twice daily). NUBEQA should be taken with food. Each tablet should be swallowed whole.
Inform patients that in the event of a missed daily dose of NUBEQA, to take any missed dose, as soon as they remember prior to the next scheduled dose, and not to take two doses together to make up for a missed dose [see Dosage and Administration (2.1)].
Infertility
Advise male patients that NUBEQA may impair fertility [see Use in Specific Populations (8.3)].
Manufactured by: Orion Corporation, Orion Pharma, FI-02101 Espoo, Finland
Manufactured for: Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981 USA
© 2019 Bayer HealthCare Pharmaceuticals Inc.
For more information, call Bayer HealthCare Pharmaceuticals Inc. at Bayer at 1-888-842-2937 or go to
www.NUBEQA-us.com
Patient Package Insert
|
PATIENT INFORMATION
|
|
|
What is NUBEQA?
It is not known if NUBEQA is safe and effective in females. It is not known if NUBEQA is safe and effective in children. |
|
|
Before taking NUBEQA, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. NUBEQA may affect the way other medicines work and other medicines may affect how NUBEQA works. You should not start or stop any medicine before you talk with the healthcare provider that prescribed NUBEQA. Know the medicines you take. Keep a list of them with you to show to your healthcare provider and pharmacist when you get a new medicine. |
|
|
How should I take NUBEQA?
|
|
|
What are the possible side effects of NUBEQA? NUBEQA may cause serious side effects, including:
|
|
|
The most common side effects of NUBEQA in people with nmCRPC include: |
|
|
|
|
The most common side effects of NUBEQA when used in combination with docetaxel in people with mHSPC include: |
|
|
|
|
NUBEQA may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility. These are not all the possible side effects of NUBEQA. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
|
How should I store NUBEQA?
Keep NUBEQA and all medicines out of the reach of children. |
|
|
General information about the safe and effective use of NUBEQA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NUBEQA for a condition for which it was not prescribed. Do not give NUBEQA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about NUBEQA that is written for health professionals. |
|
|
What are the ingredients in NUBEQA?
Manufactured by: Orion Corporation, Orion Pharma, FI-02101 Espoo, Finland Manufactured for: Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981 USA © 2019 Bayer HealthCare Pharmaceuticals Inc. For more information, call Bayer HealthCare Pharmaceuticals Inc. at Bayer at 1-888-842-2937 or go to www.NUBEQA-us.com |
|
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 10/2023