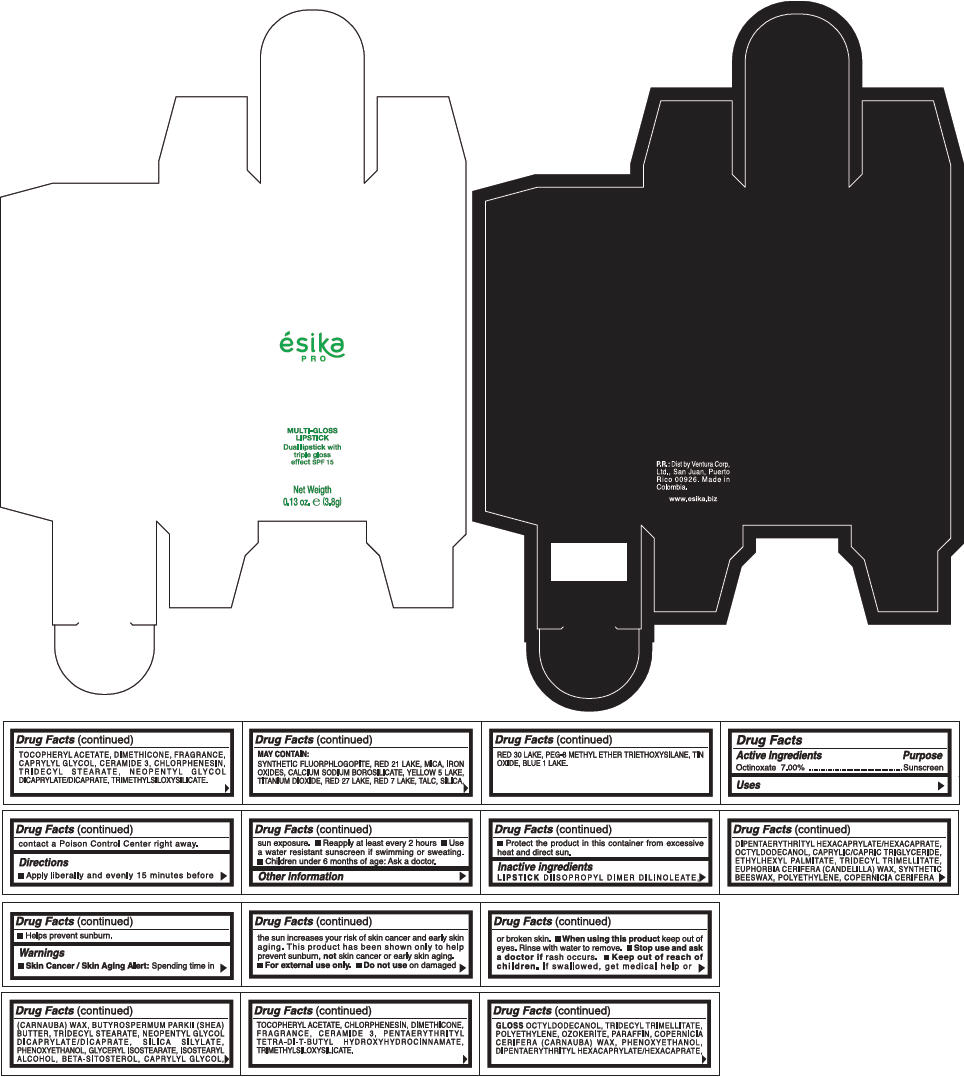
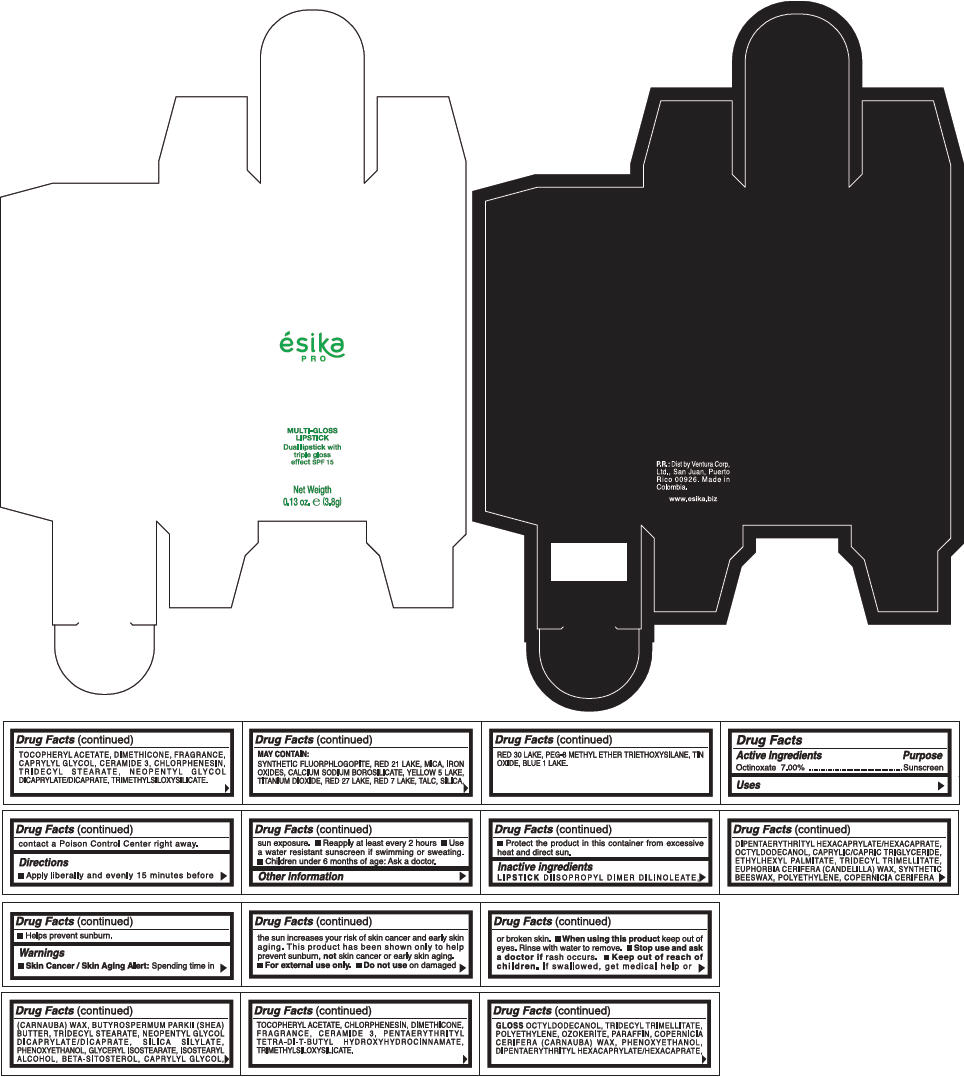
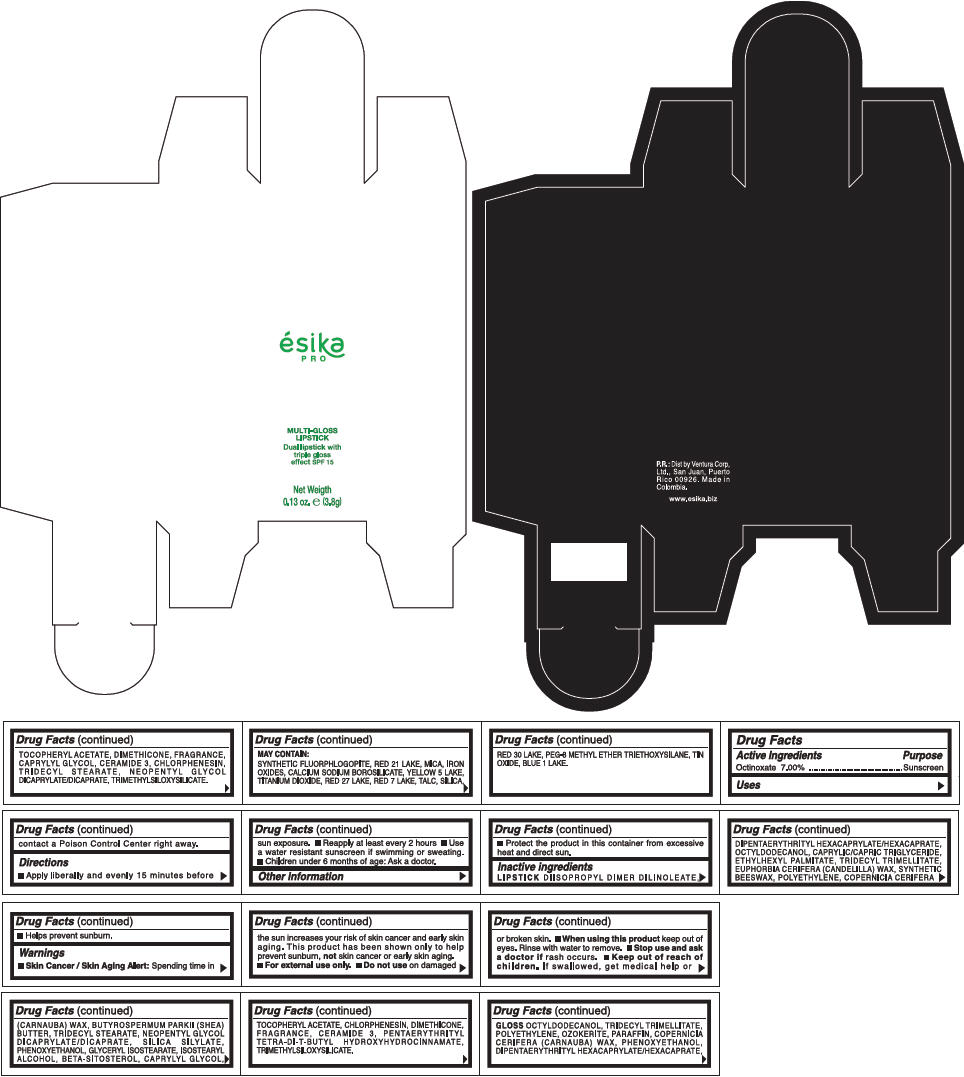
Warnings
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months of age: Ask a doctor
Inactive ingredients
LIPSTICK
DIISOPROPYL DIMER DILINOLEATE, DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE, OCTYLDODECANOL, CAPRYLIC/CAPRIC TRIGLYCERIDE, ETHYLHEXYL PALMITATE, TRIDECYL TRIMELLITATE, EUPHORBIA CERIFERA (CANDELILLA) WAX, SYNTHETIC BEESWAX, POLYETHYLENE, COPERNICIA CERIFERA (CARNAUBA) WAX, BUTYROSPERMUM PARKII (SHEA) BUTTER, TRIDECYL STEARATE, NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE, SILICA SILYLATE, PHENOXYETHANOL, GLYCERYL ISOSTEARATE, ISOSTEARYL ALCOHOL, BETA-SITOSTEROL, CAPRYLYL GLYCOL, TOCOPHERYL ACETATE, CHLORPHENESIN, DIMETHICONE, FRAGRANCE, CERAMIDE 3, PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE, TRIMETHYLSILOXYSILICATE.
GLOSS
OCTYLDODECANOL, TRIDECYL TRIMELLITATE, POLYETHYLENE, OZOKERITE, PARAFFIN, COPERNICIA CERIFERA (CARNAUBA) WAX, PHENOXYETHANOL, DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE, TOCOPHERYL ACETATE, DIMETHICONE, FRAGRANCE, CAPRYLYL GLYCOL, CERAMIDE 3, CHLORPHENESIN, TRIDECYL STEARATE, NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE, TRIMETHYLSILOXYSILICATE.
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (FUCSIA) - PURPLE
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
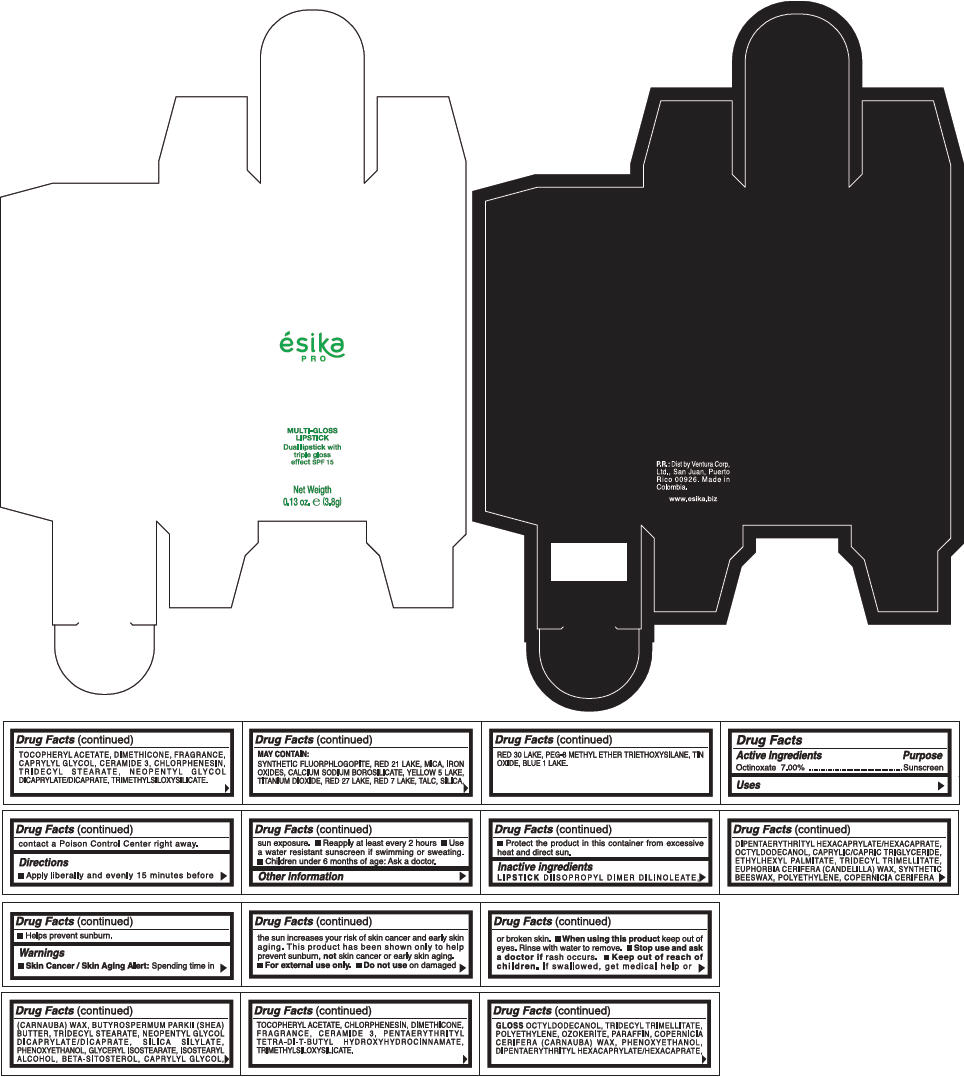
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (CEREZA) - RED
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
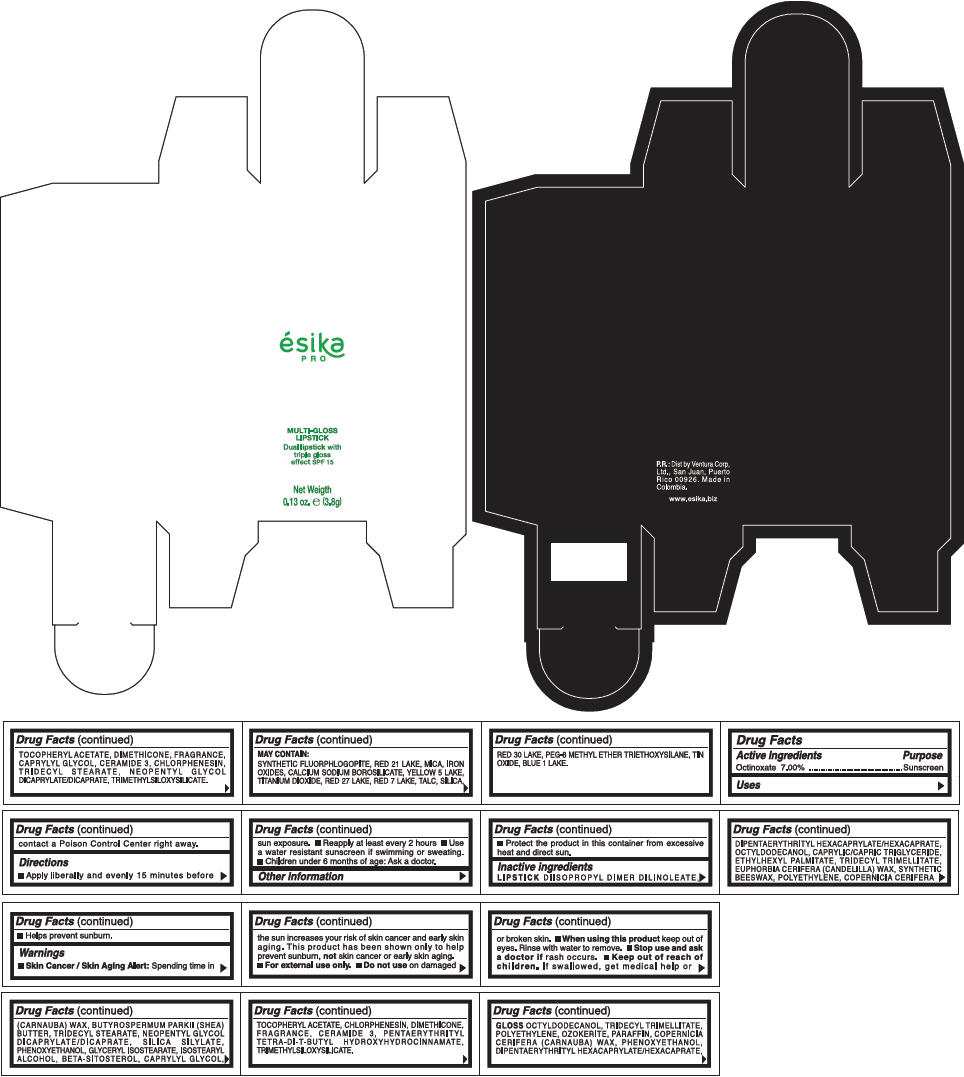
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (ROSA) - RED
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
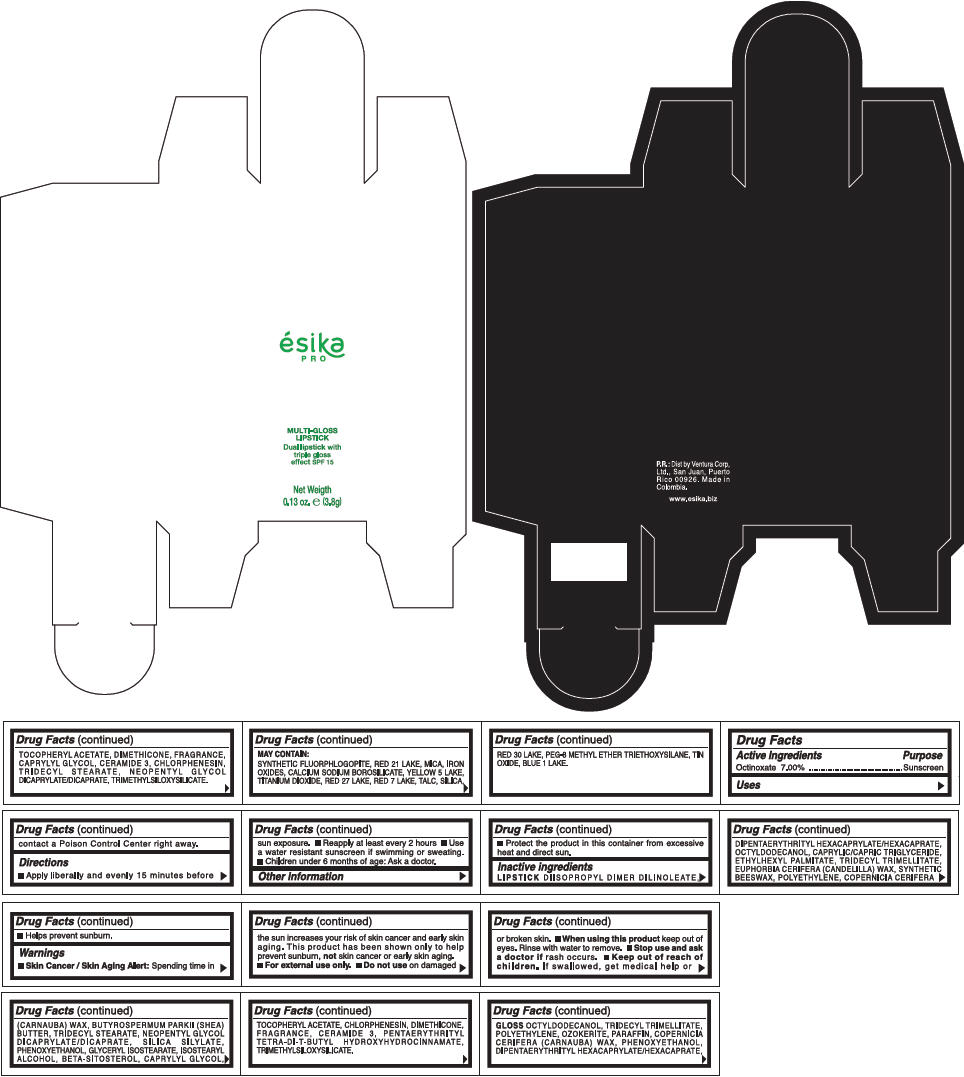
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (RUBI) - RED
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
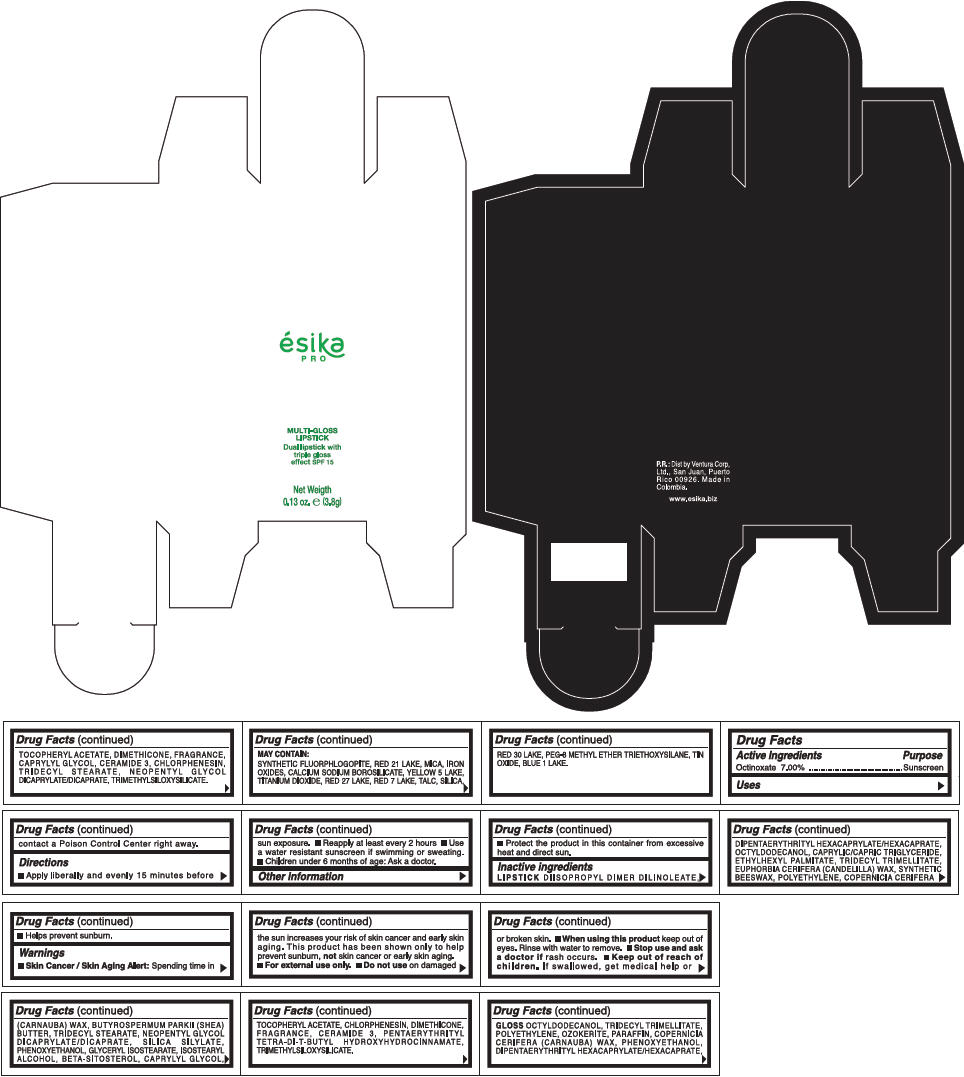
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (AMBAR) - BROWN
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
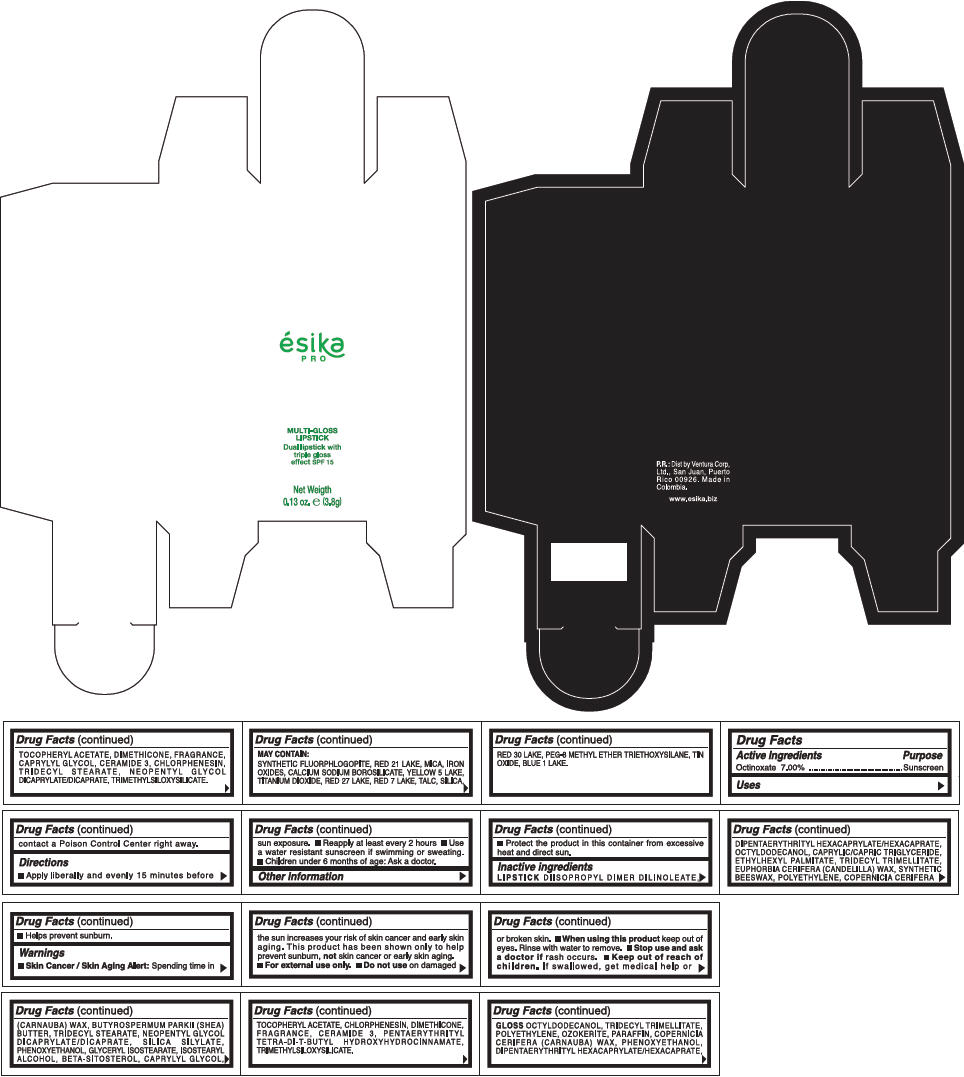
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (FRESA) - RED
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
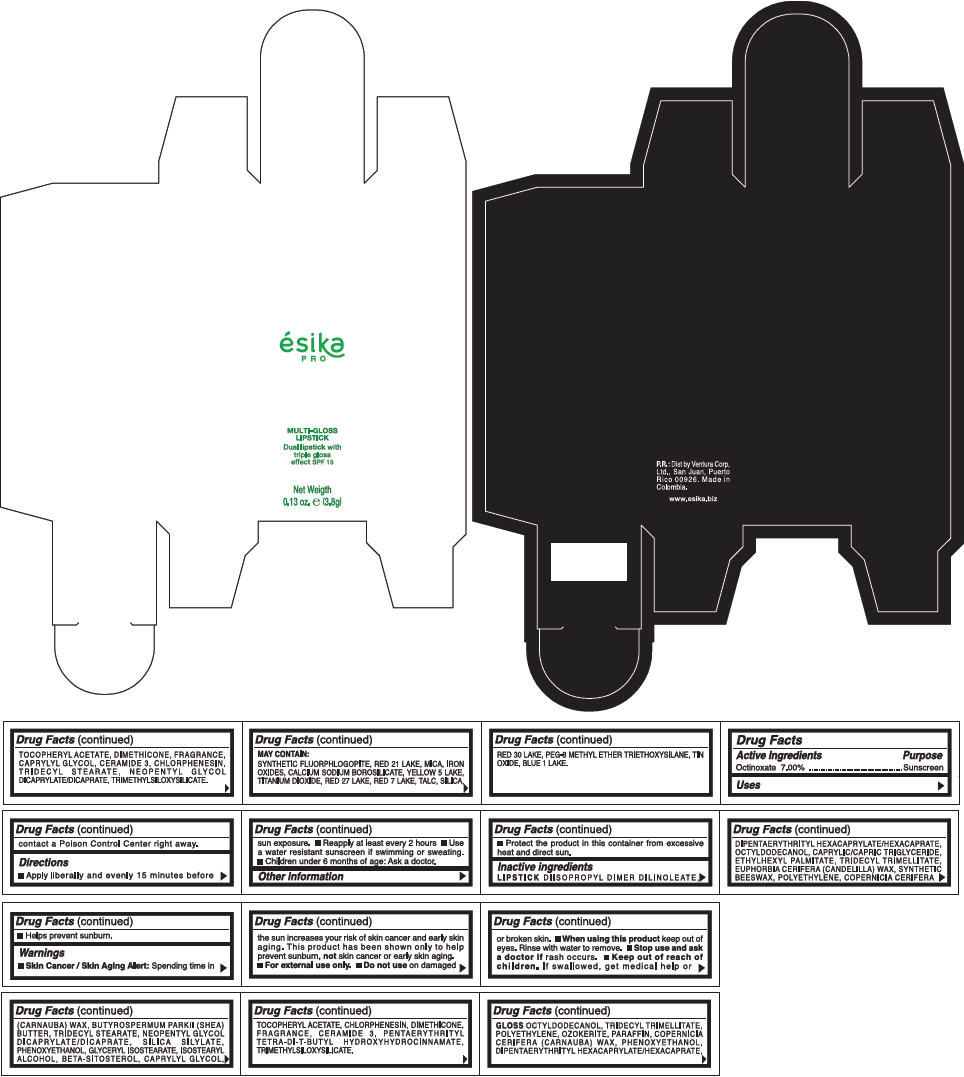
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (CHAMPAGE) - BEIGE
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
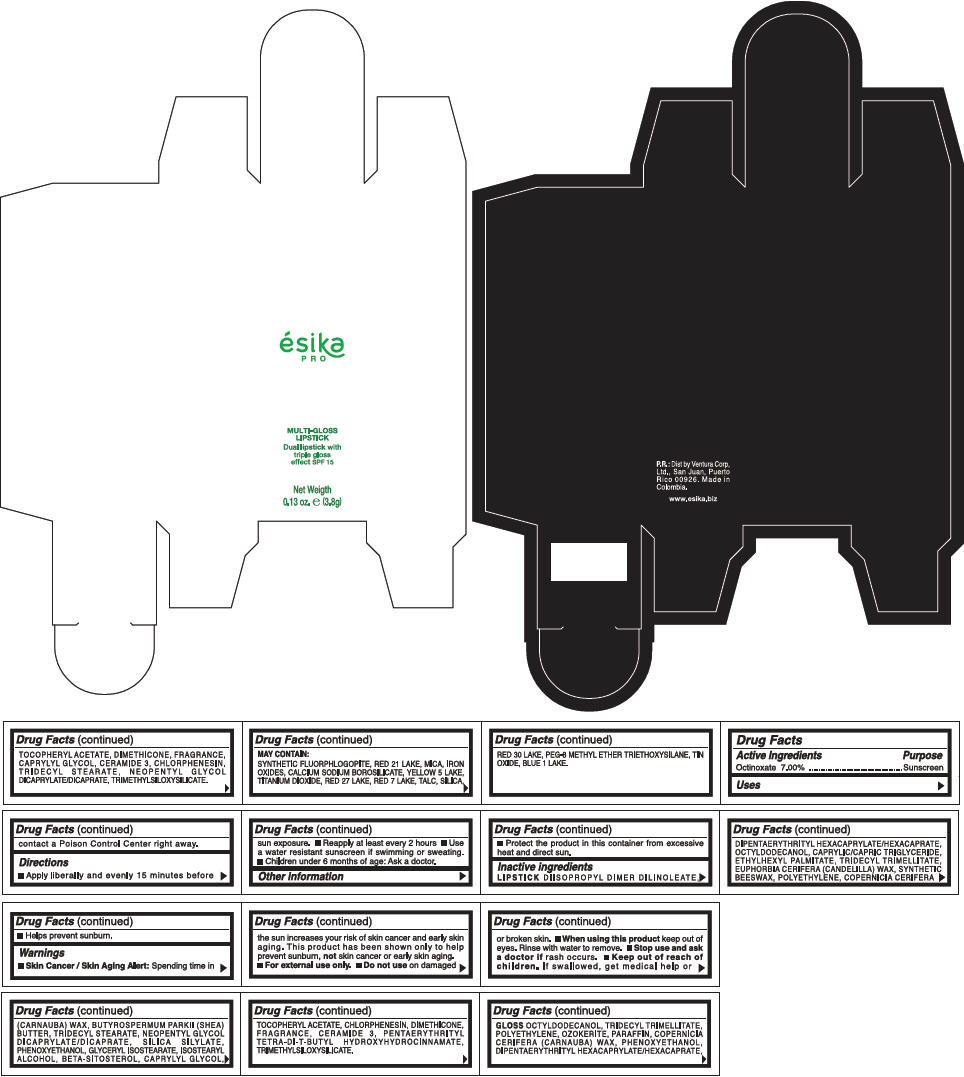
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (COBRE) - ORANGE
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
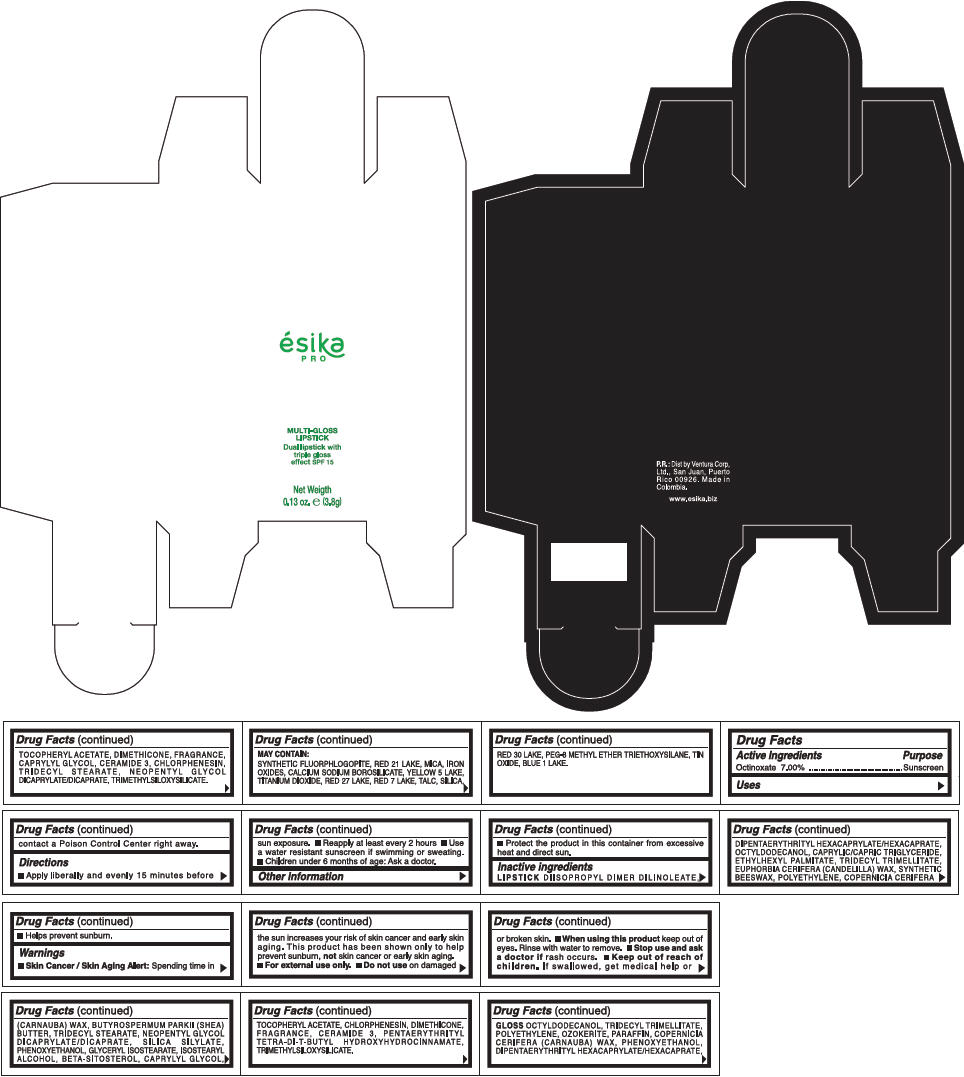
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (PIMIENTA CALIENTE) - BROWN
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)
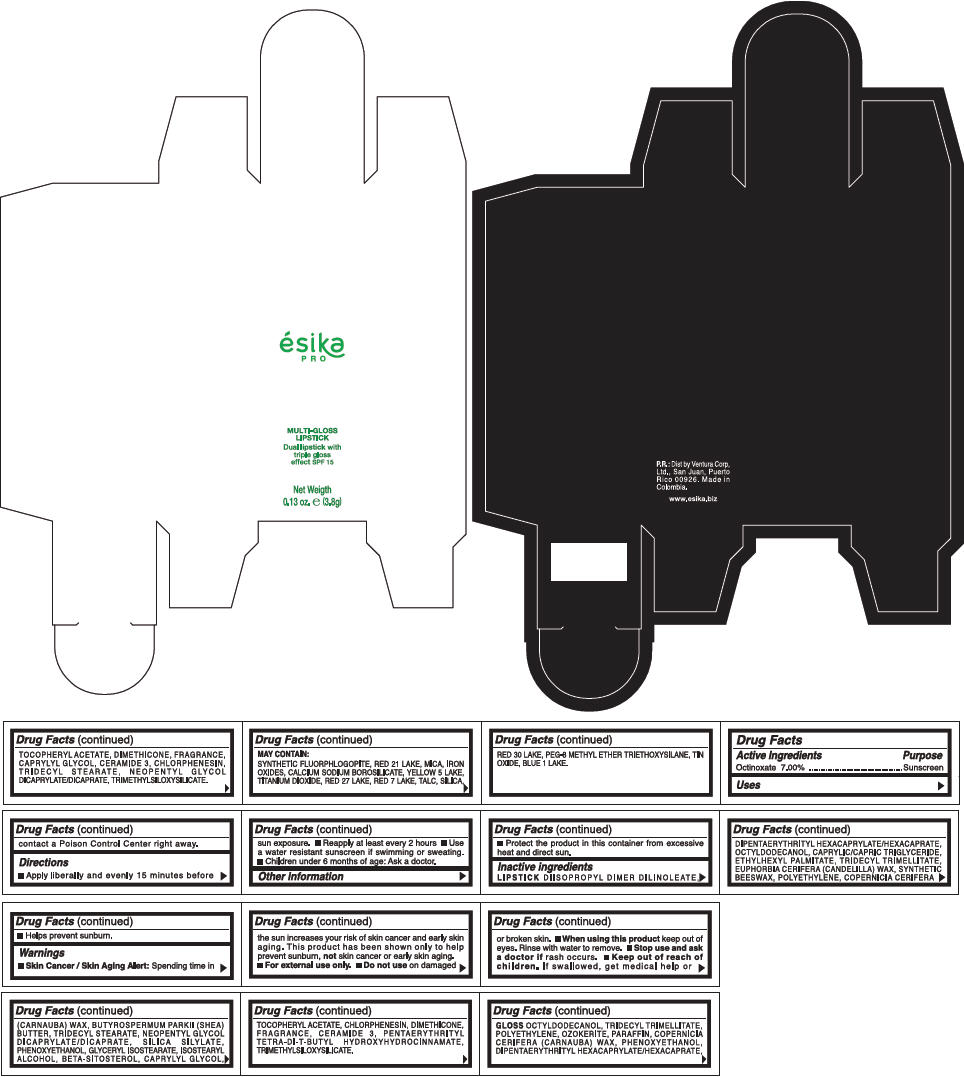
PRINCIPAL DISPLAY PANEL - 3.8 g Tube Box - (DIAMANTE ROSA) - PINK
ésika
PRO
MULTI-GLOSS
LIPSTICK
Dual lipstick with
triple gloss
effect SPF 15
Net Weigth
0.13 oz. e (3.8g)