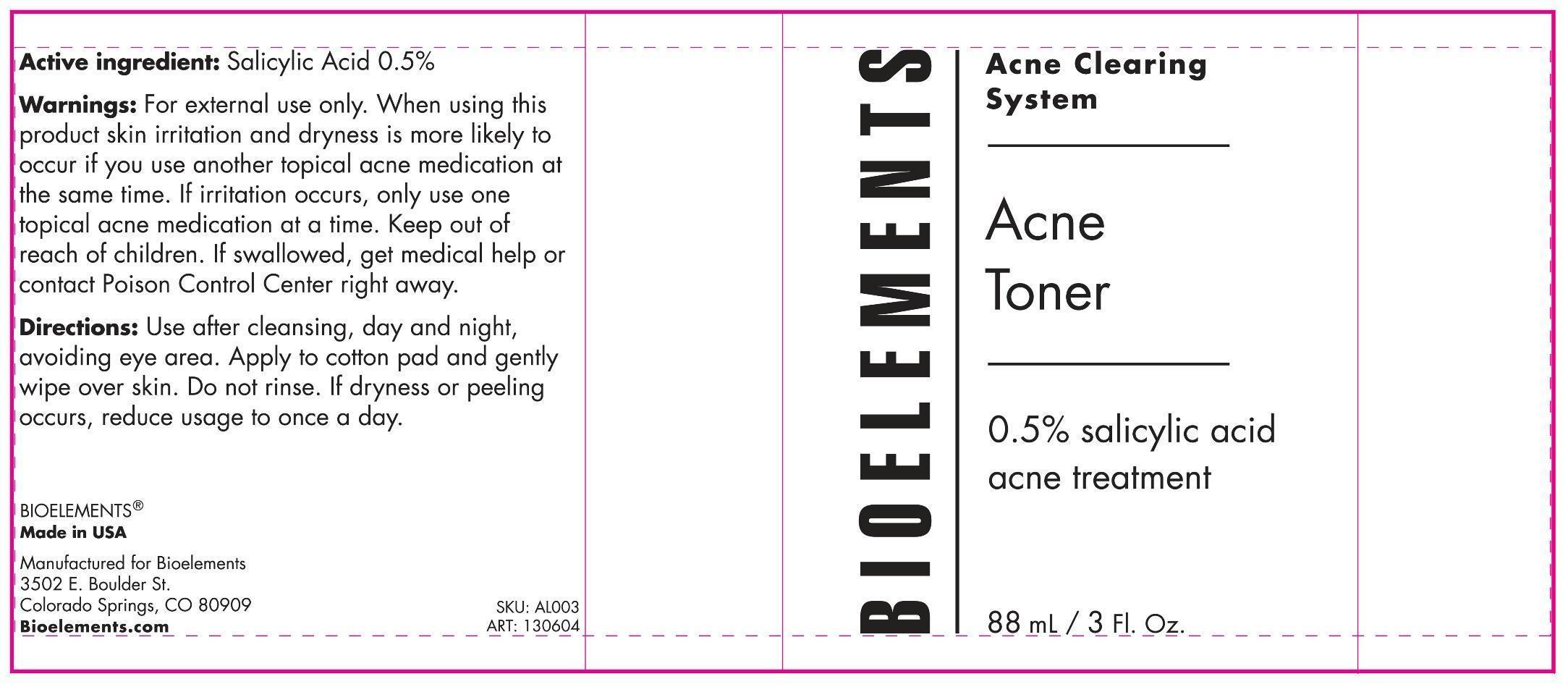
Active ingredient Purpose
Salicylic Acid 0.5% Acne treatment
Uses
For the treatment of acne
Keep out of reach of children. If swallowed get medical help or contact Poison Control Center right away.
When using this product: Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
warnings
For external use only
Directions
Use after cleansing, day and night, avoiding eye area. Apply to cotton pad and gently wipe over skin. Do not rinse. If dryness or peeling occurs, reduce usage to once a day.
Inactive ingredients
Water (Aqua, Eau), Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract, SD Alcohol, Pentylene Glycol, Hexylene Glycol, PEG-40 Hydrogenated Castor Oil, Glycerin, Vitis Vinifera (Grape) Fruit Extract, Mel (Honey) Extract (Miel Extrait de mile), Sodium Hydroxide, Ananas Sativus (Pineapple) Fruit Extract, Citrus Medica Limonium (Lemon) Fruit Extract, Citrus Aurantifolia (Lime) Fruit Extract, Lavandula Angustifolia(Lavender) Extract, Citrus Grandis (Grapefruit) Fruit Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Carica Papaya (Papaya) Fruit Extract, Citrus Grandis (Grapefruit) Peel Oil, Canarium Luzonicum Gum Nonvolatiles, Citrus Aurantium Dulcis (Orange) Oil, Caprylic/Capric Triglyceride, Lavandula Hybrida (Lavandin) Oil, Tetrahexyldecyl Ascorbate, Ethylhexylglycerin, Phenoxyethanol.