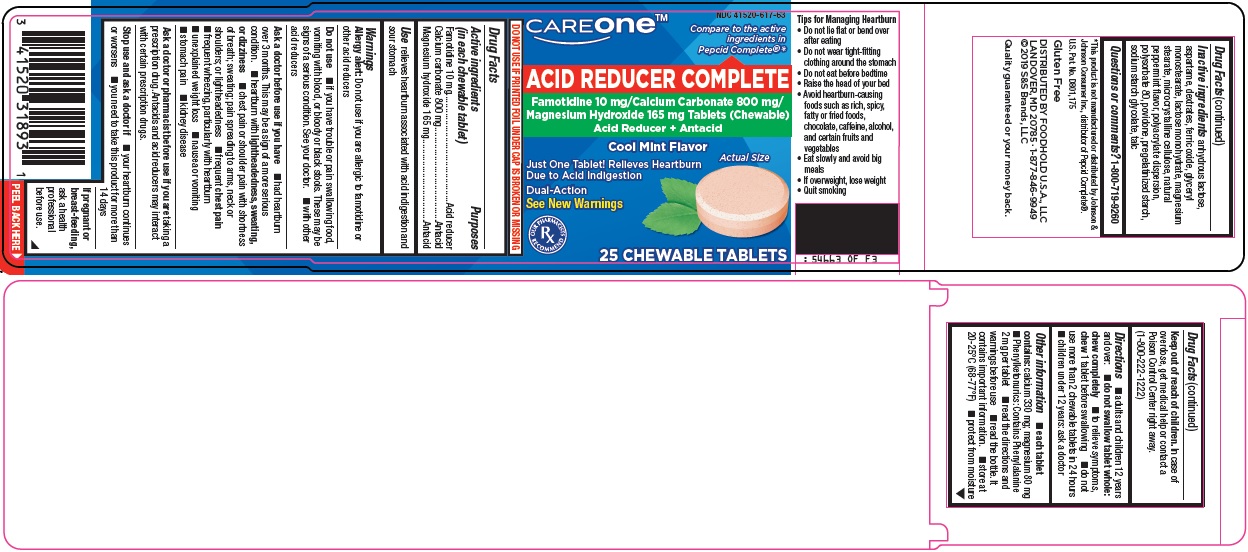
Active ingredients (in each chewable tablet)
Famotidine 10 mg
Calcium carbonate 800 mg
Magnesium hydroxide 165 mg
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
- •
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- •
- with other acid reducers
Ask a doctor before use if you have
- •
- had heartburn over 3 months. This may be a sign of a more serious condition.
- •
- heartburn with lightheadedness, sweating, or dizziness
- •
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- •
- frequent chest pain
- •
- frequent wheezing, particularly with heartburn
- •
- unexplained weight loss
- •
- nausea or vomiting
- •
- stomach pain
- •
- kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Antacids and acid reducers may interact with certain prescription drugs.
Directions
- •
- adults and children 12 years and over:
- •
- do not swallow tablet whole: chew completely
- •
- to relieve symptoms, chew 1 tablet before swallowing
- •
- do not use more than 2 chewable tablets in 24 hours
- •
- children under 12 years: ask a doctor
Other information
- •
- each tablet contains: calcium 330 mg; magnesium 80 mg
- •
- Phenylketonurics: Contains Phenylalanine 2 mg per tablet
- •
- read the directions and warnings before use
- •
- read the bottle. It contains important information.
- •
- store at 20-25˚C (68-77˚F)
- •
- protect from moisture
Inactive ingredients
anhydrous lactose, aspartame, dextrates, ferric oxide, glyceryl monostearate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, natural peppermint flavor, polyacrylate dispersion, polysorbate 80, povidone, pregelatinized starch, sodium starch glycolate, talc
Principal Display Panel
CAREONE™
Compare to the active ingredients in Pepcid Complete®
ACID REDUCER COMPLETE
Famotidine 10 mg/Calcium Carbonate 800 mg/Magnesium Hydroxide 165 mg Tablets (Chewable)
Acid Reducer + Antacid
Cool Mint Flavor
Actual Size
Just One Tablet! Relieves Heartburn Due to Acid Indigestion
Dual-Action
See New Warnings
OUR PHARMACISTS RECOMMEND
25 CHEWABLE TABLETS