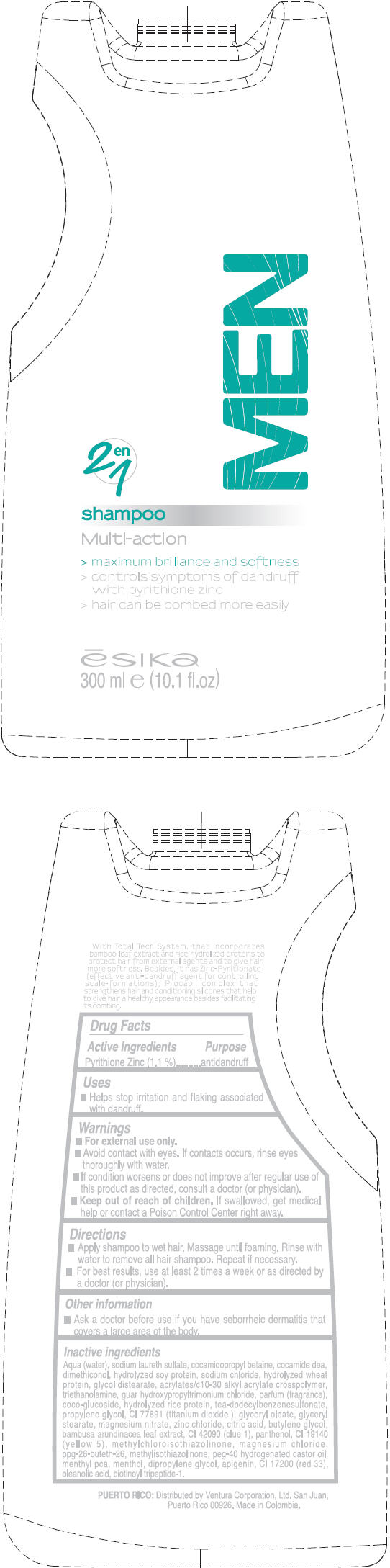
Warnings
- for external use only.
Directions
- Apply shampoo to wet hair. Massage until foaming. Rinse with water to remove all hair shampoo. Repeat if necessary.
- For best results, use at least 2 times a week or as directed by a doctor (or physician).
Other information
- Ask a doctor before use if you have seborrheic dermatitis that covers a large area of the body.
Inactive ingredients
Aqua (water), sodium laureth sulfate, cocamidopropyl betaine, cocamide dea, dimethiconol, hydrolyzed soy protein, sodium chloride, hydrolyzed wheat protein, glycol distearate, acrylates/c10-30 alkyl acrylate crosspolymer, triethanolamine, guar hydroxypropyltrimonium chloride, parfum (fragrance), coco-glucoside, hydrolyzed rice protein, tea-dodecylbenzenesulfonate, propylene glycol, CI 77891 (titanium dioxide ), glyceryl oleate, glyceryl stearate, magnesium nitrate, zinc chloride, citric acid, butylene glycol, bambusa arundinacea leaf extract, CI 42090 (blue 1), panthenol, CI 19140 (yellow 5), methylchloroisothiazolinone, magnesium chloride, ppg-26-buteth-26, methylisothiazolinone, peg-40 hydrogenated castor oil, menthyl pca, menthol, dipropylene glycol , apigenin, CI 17200 (red 33), oleanolic acid, biotinoyl tripeptide-1.