Active Ingredient
Dimethicone 1.25 percent
Use
-
Helps prevent and temporarily protect dry, cracked, chafed skin.
Warnings
For external use only
When using this product do not get into eyes
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- Apply liberally as often as needed.
Inactive Ingredients
Water, Mineral Oil, Propylene Glycol, Glyceryl Distearate, Glyceryl Stearate, Microcrystalline Wax, Cetyl Alcohol, Glycerin, Aloe Barbadensis Leaf Juice, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polysorbate 20, Simmondsia Chinensis (Jojoba) Seed Oil, Tetrahydroxypropyl Ethylenediamine, Phenoyethanol, Fragrance, Methylparaben, Benzatriazoyl Dodecyl p-Cresol, Ethylparaben, Butylparaben, Propylparaben, Isobutylparaben
Active ingredient
Alcohol 67 percent
Use
Hand sanitizer to decrease bacteria on the skin that potentially can cause disease
Warnings
Flammable. Keep away from fire or flame.
For external use only.
When using this product, do not use in the eyes.
Discontinue use and ask a doctor if
-
irritate and redness develop.
-
condition persists for more than 72 hours.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Wet hands thoroughly with product and allow to dry without wiping.
Other Information
Store in a cool, dry place below 104 deg F (40 deg C).
Inactive ingredients
Water, Isopropyl Alcohol, Glycerin, Glyceryl Laurate.
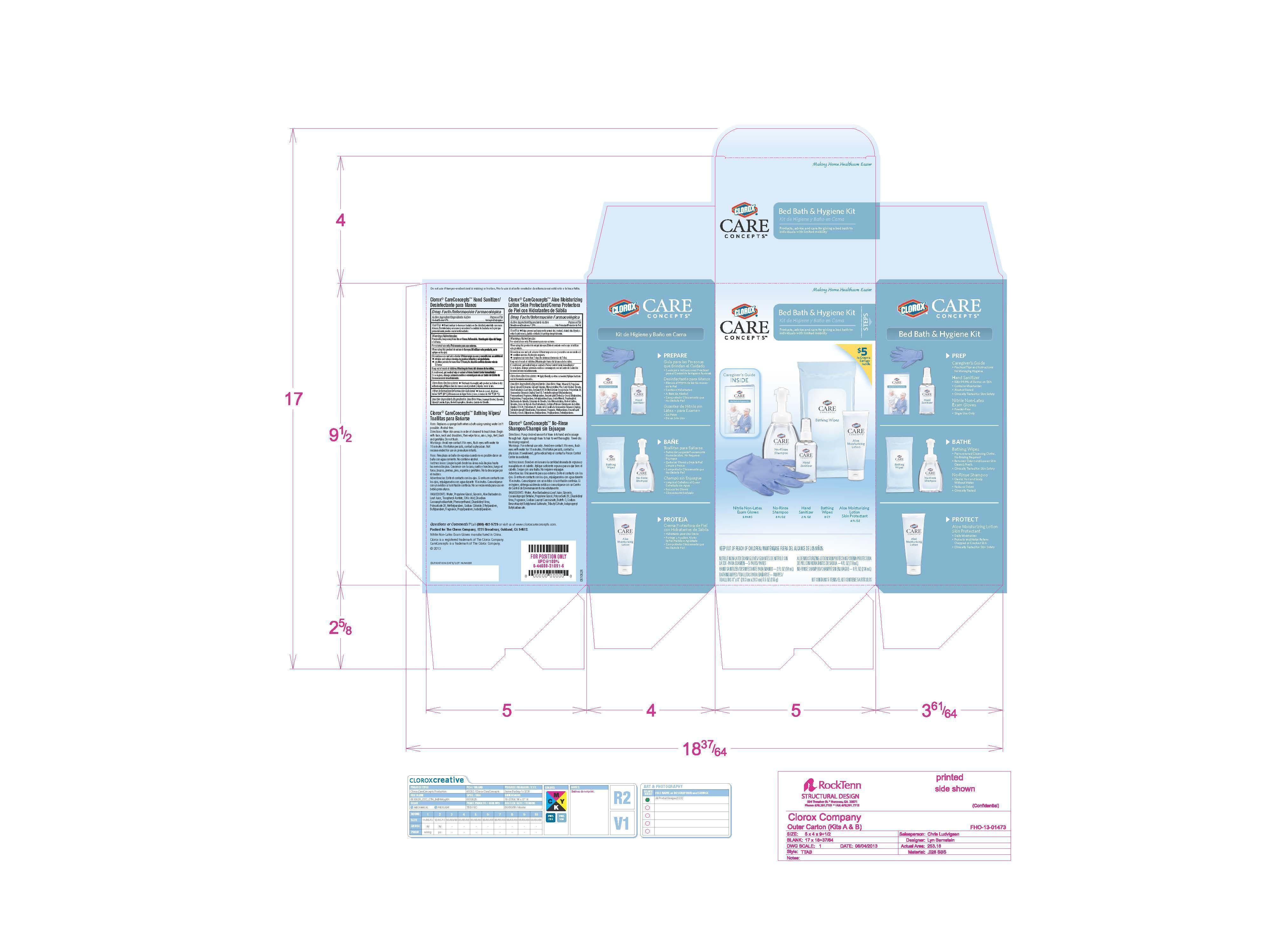 Clorox Care Concepts
Clorox Care Concepts
Bed Bath and Hygiene Kit
Nitrile Non-Latex Exam Gloves
No-Rinse Shampoo
Hand Sanitizer
Bathing Wipes
Aloe Moisturizing Lotion Skin Protectant
 Clorox Care Concepts
Clorox Care Concepts