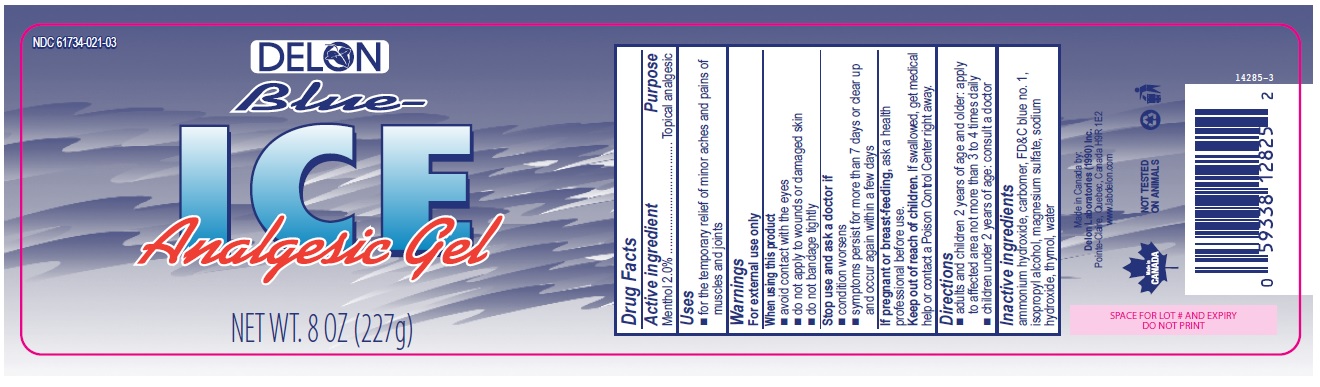
Active ingredient
Menthol 2.0%
Purpose
Topical analgesic
Uses
- for the temporary relief of minor aches and pains of muscles and joints
Warnings
For external use only
When using this product
- avoid contact with the eyes
- do not apply to wounds or damaged skin
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: consult a doctor
Inactive ingredients
ammonium hydroxide, carbomer, FD&C blue no. 1, isopropyl alcohol, magnesium sulfate, sodium hydroxide, thymol, water
Delon 8oz (227g)
Penetro 227g


