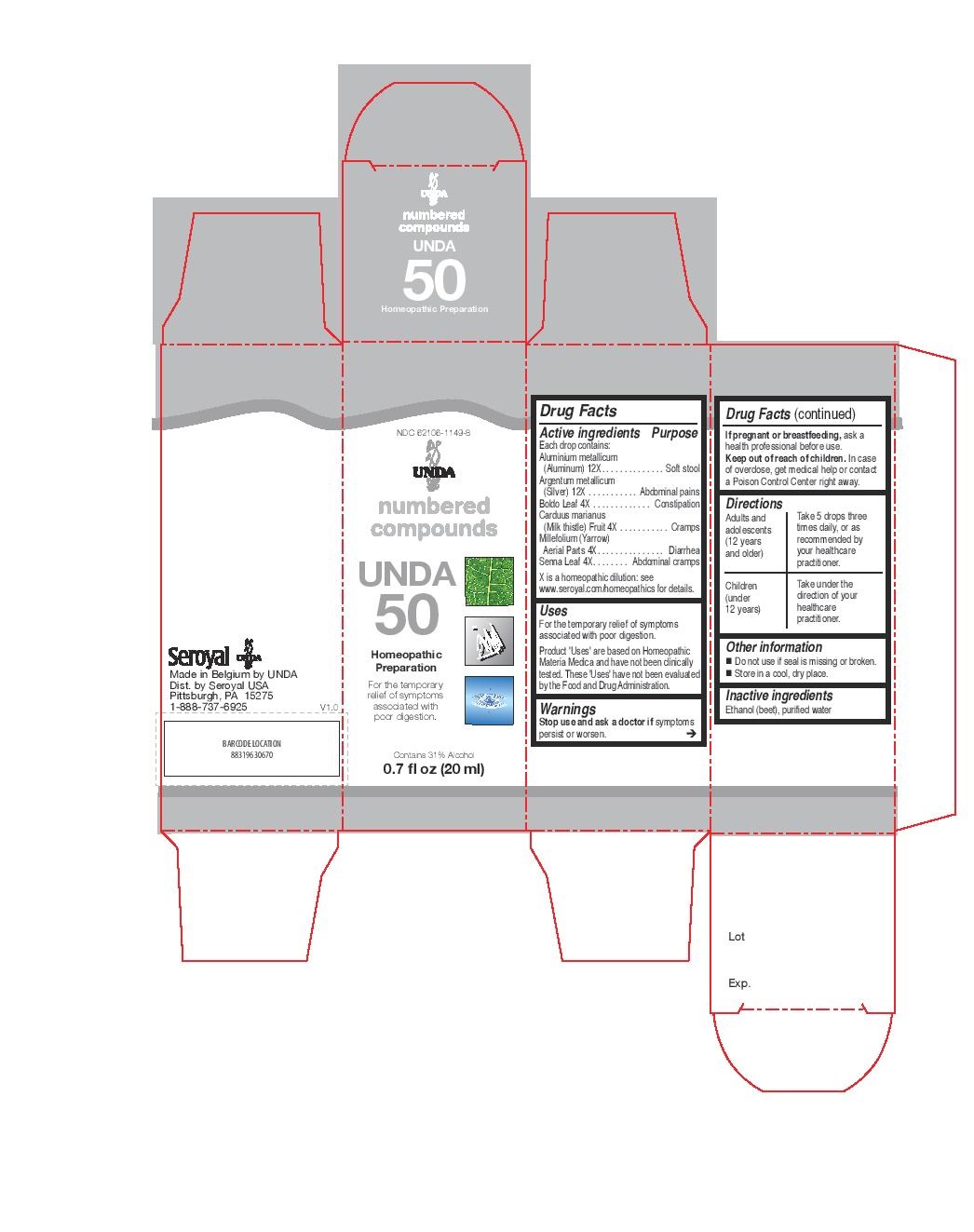
Active ingredients
Each drop contains:
Absinthium (Common wormwood) Aerial Parts 4X
Antimonium crudum (Antimonous sulfide) 12X
Argentum metallicum (Silver) 12X
Condurango Bark 4X
Jateorhiza palmata (Columbo) Root 4X
Laurus nobilis (Bay tree) Leaf 4X
Menyanthes trifoliata (Buckbean) Aerial Parts 4X
Rhamnus frangula (Buckthorn) Bark 4X
Uses
For the temporary relief of symptoms associated with stomach
acidity, gas, belching and lack of appetite.
Warnings
Stop use and ask a doctor if symptoms
persist or worsen.
If pregnant or breastfeeding, ask a
health professional before use.
Keep out of reach of children. In case
of overdose, get medical help or contact
a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
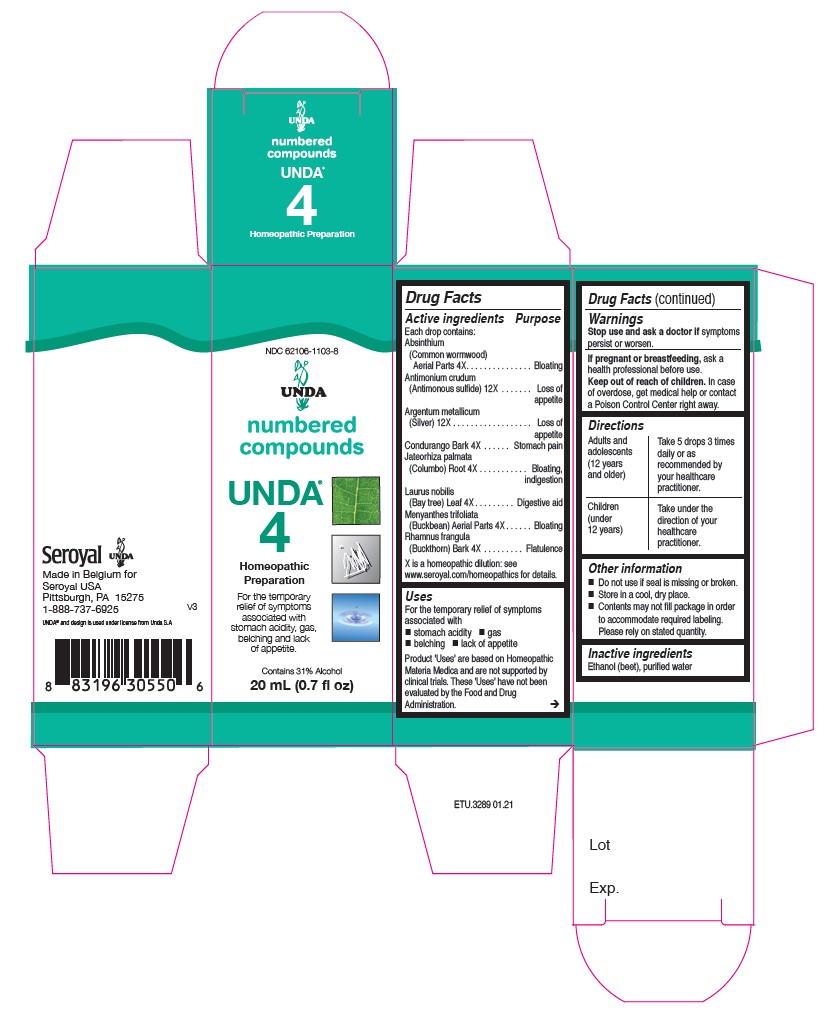
Uses
For the temporary relief of symptoms associated with
stomach acidity, gas, belching and lack of appetite.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Active ingredients
Each drop contains:
Aluminium metallicum (Aluminum) 12X
Argentum metallicum (Silver) 12X
Boldo Leaf 4X
Carduus marianus (Milk thistle) Fruit 4X
Millefolium (Yarrow) Aerial Parts 4X
Senna Leaf 4X
Warnings
Stop use and ask a doctor if symptomspersist or worsen
If you are pregnant or breastfeeding, ask a healthcare practitioner before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and Children (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Takeunder the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms associated with poor digestion.
Directions
Adults and Children (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Other information
Do not use if seal is missing or broken.
Store in a cool, dry place.
Contents may not fill package in order
to accommodate required labeling.
Please rely on stated quantity.