BERBERIS VULGARIS 200C- berberis vulgaris pellet
Paramesh Banerji Life Sciences LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
DRUG FACTS:
Active Ingredient
Berberis vulgaris 200C HPUS
Inactive Ingredients
Sucrose, Lactose
Purpose
Recurrent kidney stones formation, joint pains
Uses
Recurrent kidney stones formation, joint pains
Warnings
If pregnant or breast feeding ask a health professional before use.
Keep out of reach of children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Direction
Adult or child: Take three pills daily. Leaving a gap of 30 minutes after any food or as advised by your physician.
Principal Display Panel
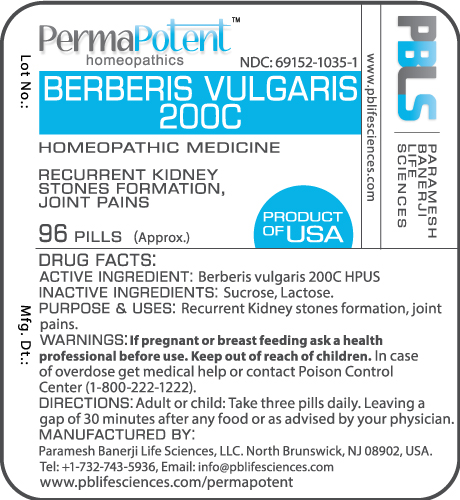
NDC: 69152-1035-1
Berberis vulgaris 200C
Homeopathic Medicine
Recurrent kidney stones formation, joint pains
96 Pills (Approx.)
Product of USA
