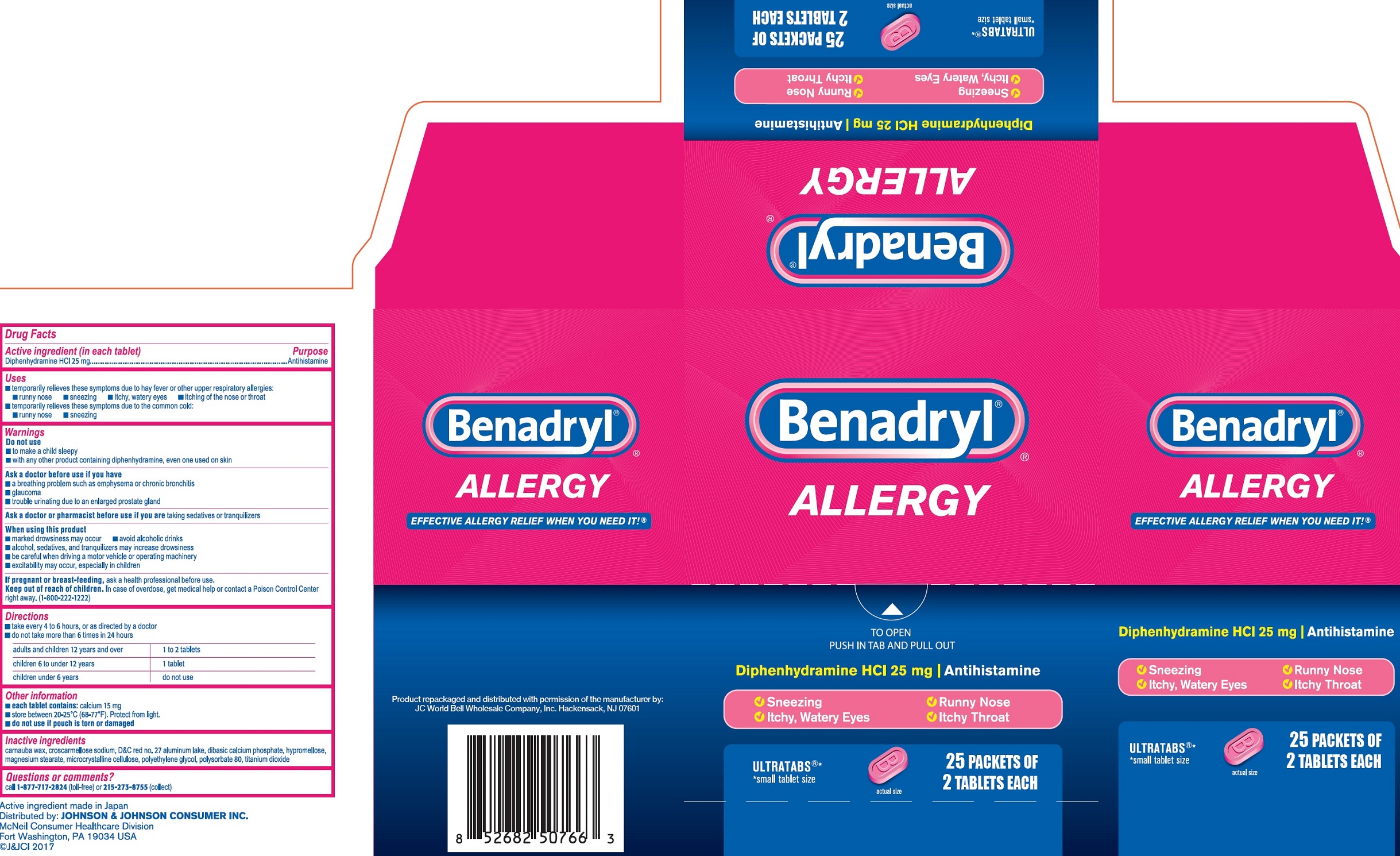
BENADRYL ULTRA TAB- diphenhydramine hydrochloride tablet, film coated
JC World Bell Wholesale Co., Inc.
----------
Active ingredient (in each tablet)
Diphenhydramine HCl 25 mg
Uses
• temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: • runny nose • sneezing • itchy, watery eyes • itching of the nose or throat • temporarily relieves these symptoms due to the common cold: • runny nose • sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
| adults and children 12 years and over | 1 to 2 tablets |
| children 6 to under 12 years | 1 tablet |
| children under 6 years | do not use |
Other information
- each tablet contains: calcium 15 mg
- store between 20-25°C (68-77°F). Protect from light.
- do not use if pouch is torn or damaged
Inactive ingredients
carnauba wax, croscarmellose sodium, D&C red no. 27 aluminum lake, dibasic calcium phosphate, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, titanium dioxide
Questions or comments?
call 1-877-717-2824 (toll-free) or 215-273-8755 (collect)
Package Labeling:
JC World Bell Wholesale Co., Inc.
