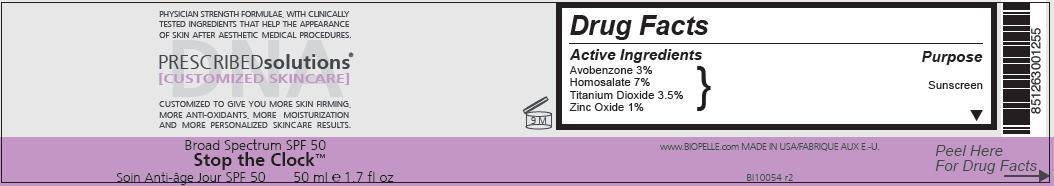
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- apply to face and under throat after cleansing
- reapply:
-
- after excessive perspiration
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF 15 or higher and other sun protection measures including:
-
- limit time in the sun, especially from 10 a.m. - 2 p.m.
-
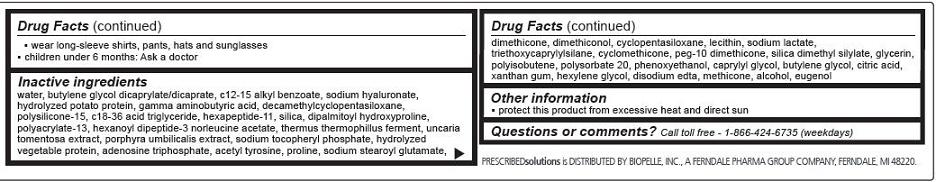
- wear long-sleeve shirts, pants, hats and sunglasses
- children under 6 months: Ask a doctor
Inactive ingredients
water, butylene glycol dicaprylate/dicaprate, c12-15 alkyl benzoate, sodium hyaluronate, hydrolyzed potato protein, gamma aminobutyric acid, decamethylcyclopentasiloxane, polysilicone-15, c18-36 acid triglyceride, hexapeptide-11, silica, dipalmitoyl hydroxyproline, polyacrylate-13, hexanoyl dipeptide-3 norleucine acetate, thermus thermophillus ferment, uncaria tomentosa extract, porphyra umbilicalis extract, sodium tocopheryl phosphate, hydrolyzed vegetable protein, adenosine triphosphate, acetyl tyrosine, proline, sodium stearoyl glutamate, dimethicone, dimethiconol, cyclopentasiloxane, lecithin, sodium lactate, triethoxycaprylylsilane, cyclomethicone, peg-10 dimethicone, silica dimethyl silylate, glycerin, polyisobutene, polysorbate 20, phenoxyethanol, caprylyl glycol, butylene glycol, citric acid, xantham gum, hexylene glycol, disodium edta, methicone, alcohol, eugenol