Active ingredients
Each drop contains:
Allium cepa (Onion) Bulb 4X
Aurum metallicum (Gold) 12X
Eucalyptus globulus (Fever tree) Leaf 4X
Inula helenium (Elecampane) Root 4X
Trigonella foenum-graecum (Fenugreek) Seed 4X
Warning:
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of mild fever.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
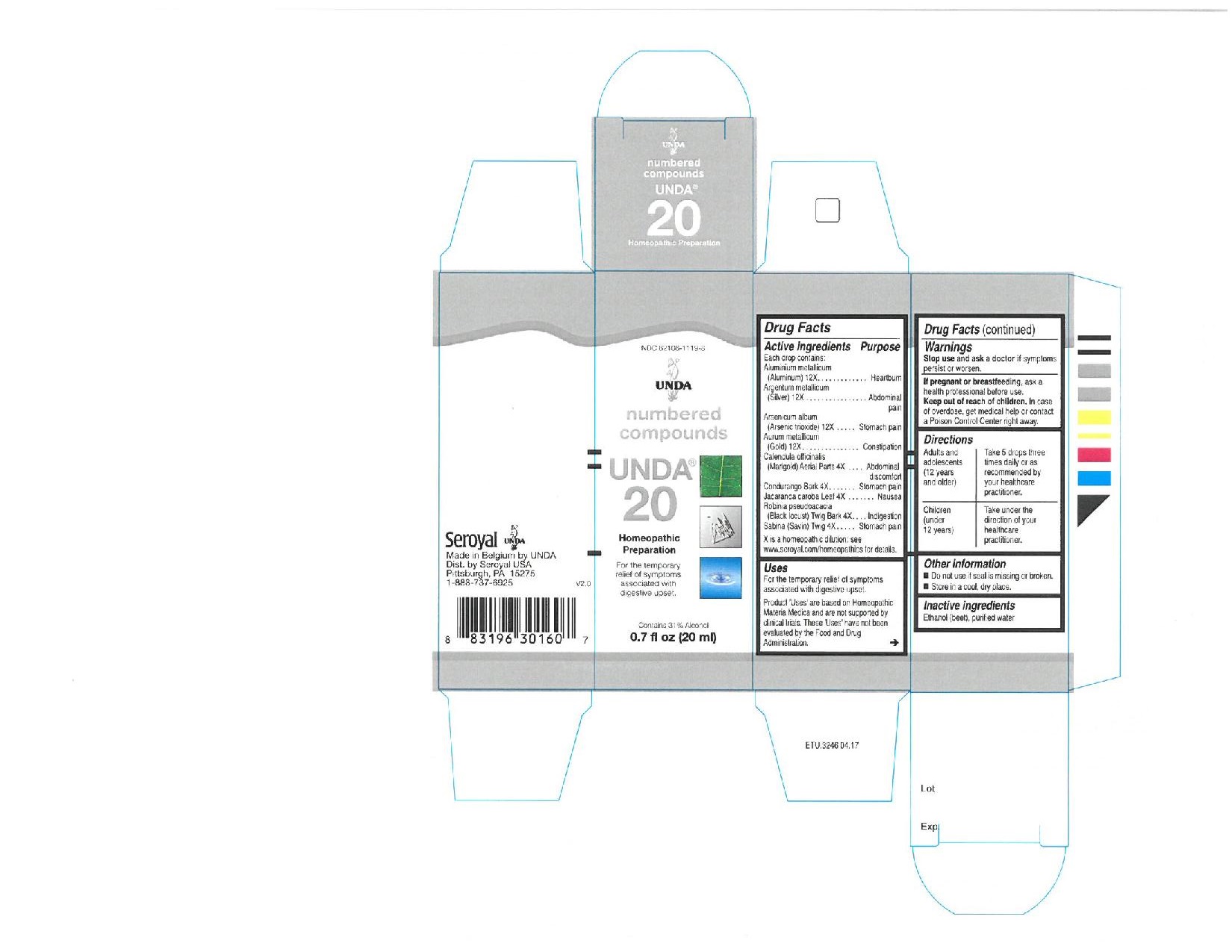
Active ingredients
Each drop contains equal parts of:
Aluminium metallicum (Aluminum) 12X
Argentum metallicum (Silver) 12X
Arsenicum album (Arsenic trioxide) 12X
Aurum metallicum (Gold) 12X
Calendula officinalis (Marigold) Flowering Tops 4X
Condurango Bark 4X
Jacaranda caroba Leaf 4X
Robinia pseudoacacia (Black locust) Twig Bark 4X
Sabina (Savin) Twig 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If you are pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms associated with digestive upset.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Each drop contains:
Aluminium metallicum (Aluminum) 12X
Aurum metallicum (Gold) 12X
Calendula officinalis (Marigold) Aerial Parts 4X
Camphora (Camphor from Cinnamomum camphora) 4X
Condurango Bark 4X
Hydrastis canadensis (Golden seal) Rhizome and Root 4X
Thuja occidentalis (White cedar) Twig 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms associated with menses and tiredness
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Active ingredients
Each drop contains:
Aurum metallicum (Gold) 12X
Capsicum annuum (Cayenne pepper) Fruit 4X
Cuprum metallicum (Copper) 12X
Pilocarpus Leaf 4X
Thymus vulgaris (Common Thyme) Aerial Parts 4X
Urtica urens (Dwarf nettle) Aerial Parts 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily, or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms associated with mild dandruff.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily, or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
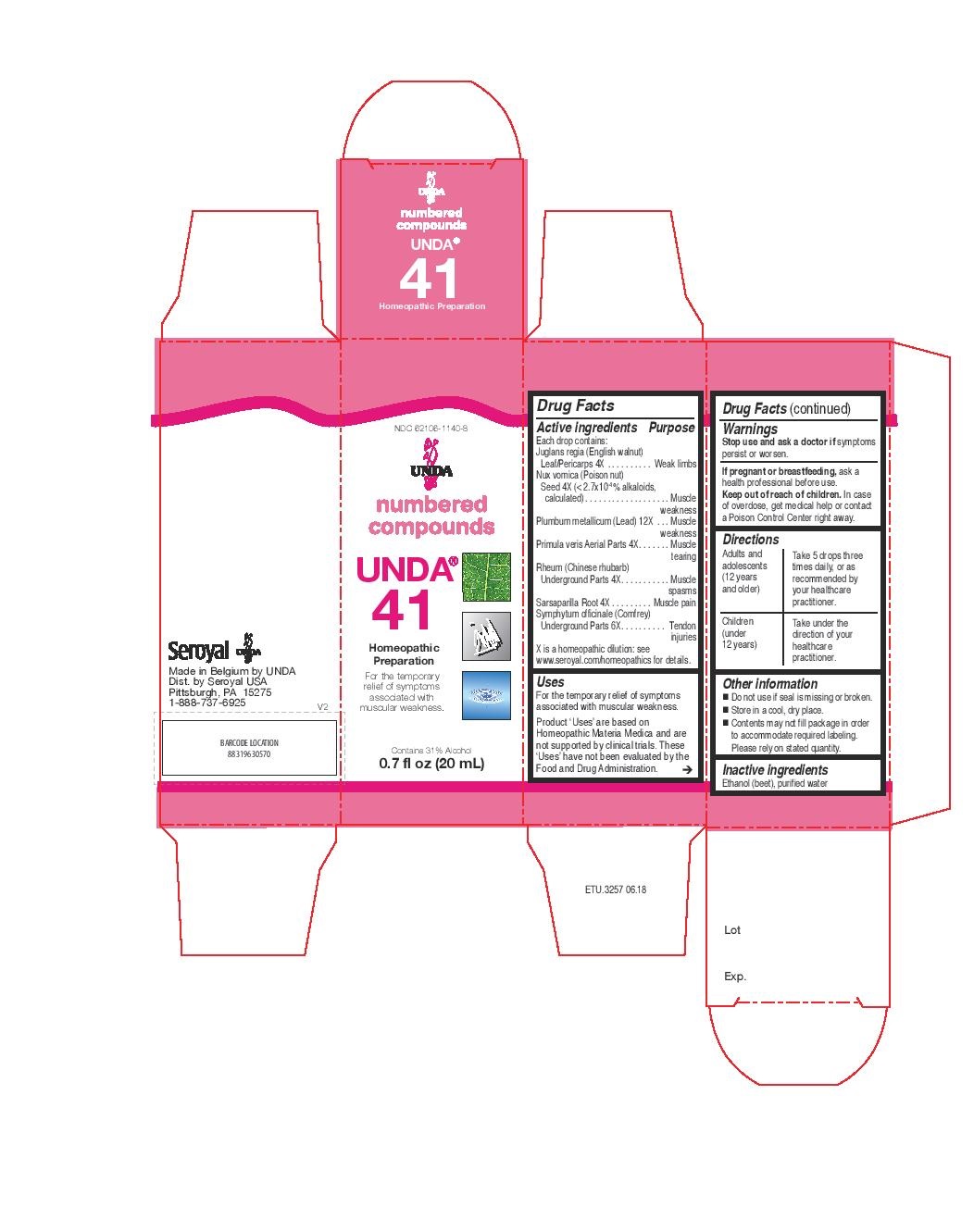
Active ingredients
Each Drop Contains:
Primula veris Whole Plant . 4X
Rheum (Chinese rhubarb) Underground Parts . 4X
Juglans regia (English walnut) Leaf/Rind/Unripe Fruit . 4X
Sarsaparilla Root . 4X
Nux vomica (Poison nut) Seed . 4X
Symphytum officinale (Comfrey) Root . 6X
Plumbum metallicum (Lead). 12X
Warnings
If you are pregnant or breastfeeding, ask a healthcare practitioner before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if symptoms persist or worsen.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily, or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the relief of symptoms associated with muscular
weakness in the elderly.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily, or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
 NDC 62106-1139-8
NDC 62106-1139-8
UNDA
numbered compounds
UNDA 40
Homeopathic Preparation
For the temporary relief of symptoms
associated with mild dandruff.
Contains 31% Alcohol
0.7 fl oz (20 ml)
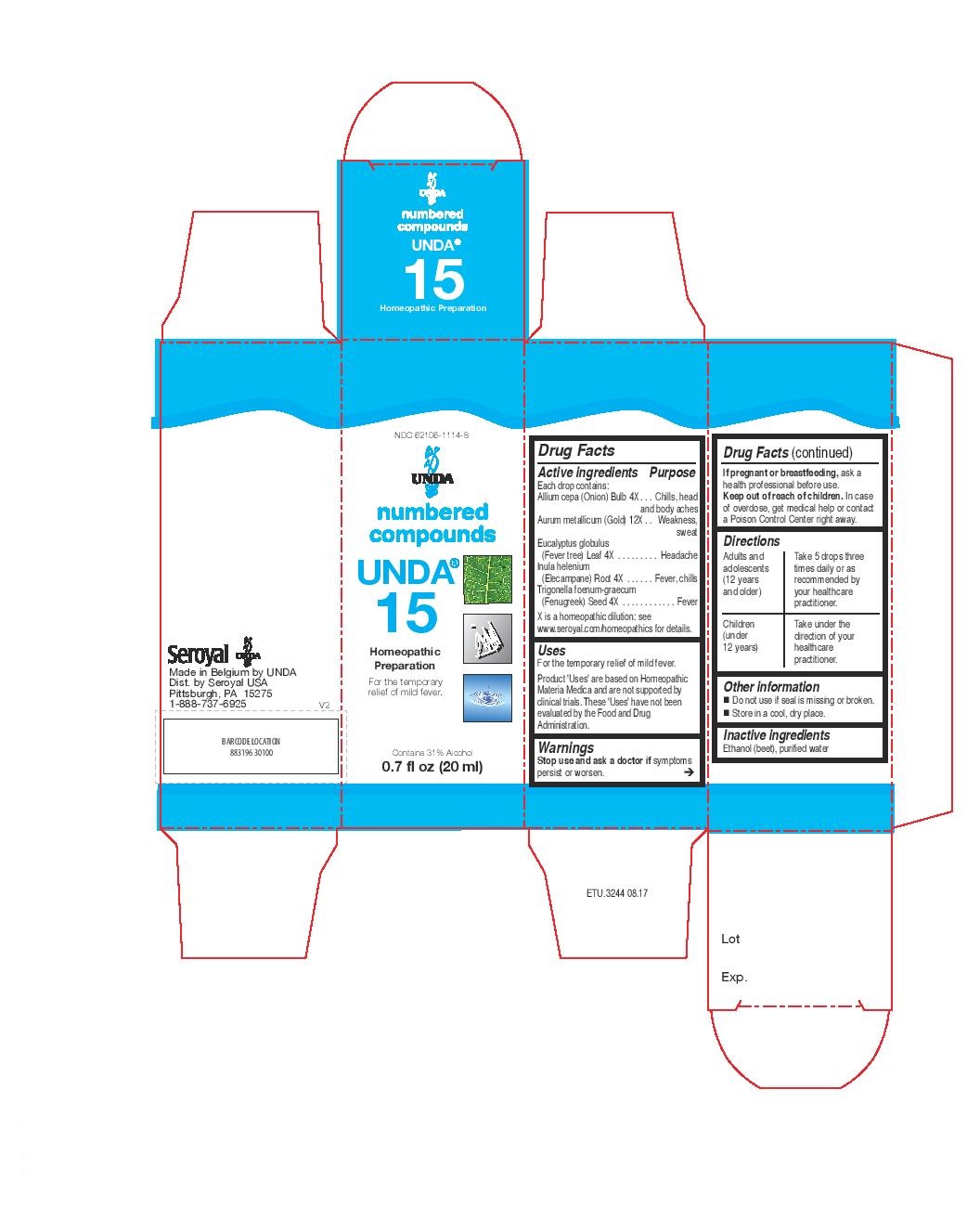
NDC 62106-1114-8
UNDA
numbered compounds
UNDA 15
Homeopathic Preparation
For the temporary relief of mild fever.
Contains 31% Alcohol
0.7 fl oz (20 ml)
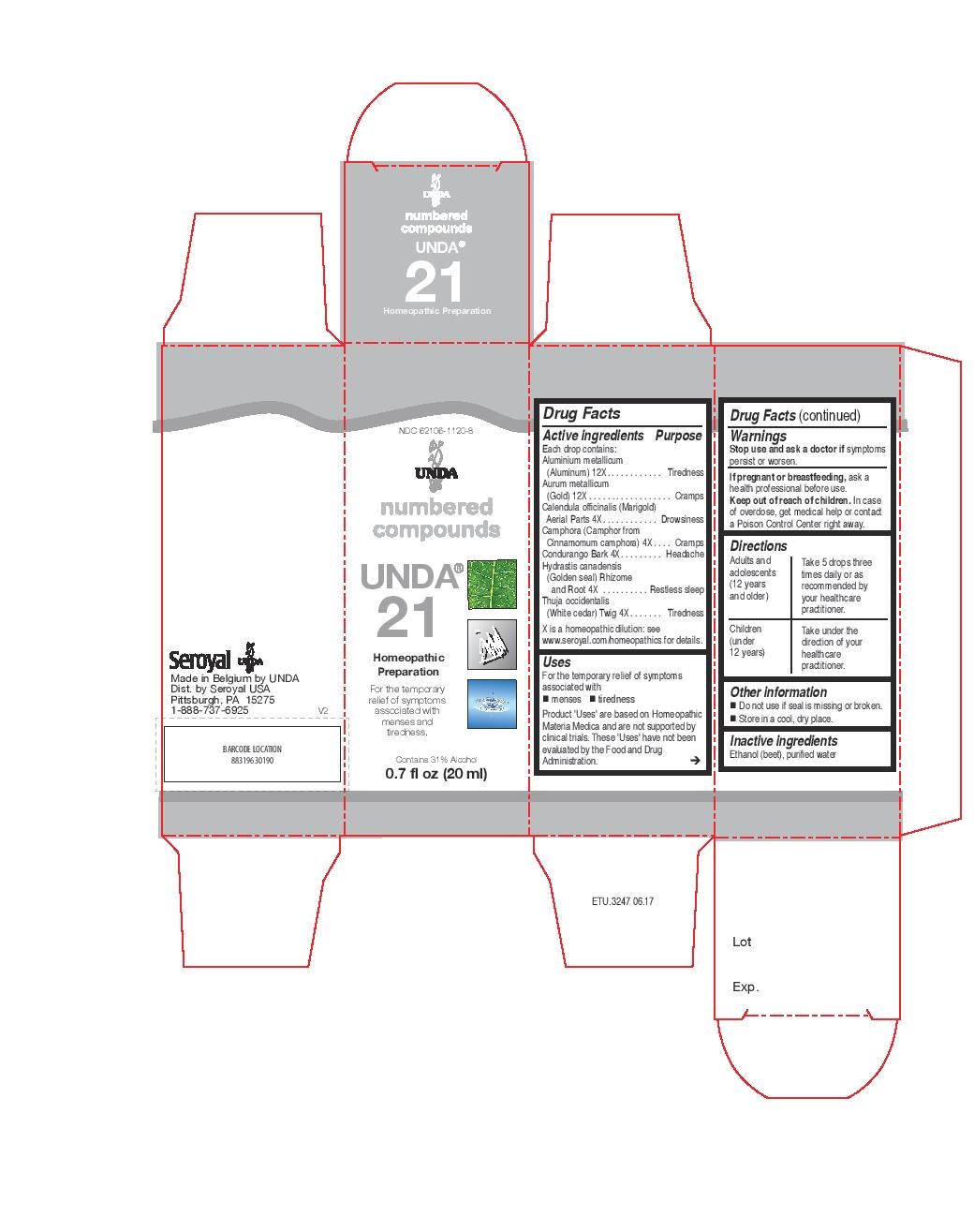
NDC 62106-1120-8
UNDA
numbered compounds
UNDA 21
Homeopathic Preparation
For the temporary relief of symptoms
associated with menses and tiredness.
Contains 31% Alcohol
0.7 fl oz (20 ml)