VETMEDIN- pimobendan capsule
Boehringer Ingelheim Animal Health USA Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Vetmedin®
1.25, 2.5, 5.0 mg
Pimobendan capsules
For Dogs
Client Information Sheet
- Client Information Sheet for Vetmedin® (pimobendan) capsules
- Replacement product for Vetmedin® (pimobendan) chewable tablets
- Contact your Veterinarian BEFORE administering this product to your dog.
This Client Information Sheet contains important information about VETMEDIN capsules, a product used as a temporary replacement for US FDA-approved VETMEDIN chewable tablets.
VETMEDIN capsules are authorized for marketing in Canada to treat congestive heart failure in dogs. You should read this information before you start giving VETMEDIN capsules and review it each time the prescription is refilled as there may be new information.
This information sheet is provided only as a summary and does not take the place of instructions from your veterinarian. If you have received this product from a pharmacy and not directly from your veterinarian, you should contact your veterinarian to inform them that you have received this replacement product. Talk with your veterinarian if you do not understand any of this information or if you want to know more about VETMEDIN capsules.
Note to prescribing veterinarians
The dose administered should be as stated in the FDA approved VETMEDIN chewable tablets package insert, accessible at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5441e5b2-cdc0-477a-bd9a-ec5f8d912281
Why did I receive VETMEDIN capsules?
Because of a shortage in the supply of the FDA-approved VETMEDIN chewable tablets, the FDA is allowing temporary use of VETMEDIN capsules in the United States.
Are VETMEDIN capsules the same as VETMEDIN chewable tablets?
- •
- The active ingredient in VETMEDIN capsules is pimobendan, just like in Vetmedin chewable tablets, the US-approved product.
- •
- Both VETMEDIN capsules and VETMEDIN chewable tablets manage the signs of congestive heart failure.
- •
- You will notice the instructions for use in the VETMEDIN capsules packaging to be slightly different than the instructions for the VETMEDIN chewable tablets. However, both VETMEDIN capsules and VETMEDIN chewable tablets contain the same amount of pimobendan and the dose should be same.
Talk to your veterinarian if you have any questions about your dog’s congestive heart failure and the use of VETMEDIN capsules.
Are there differences between VETMEDIN capsules and VETMEDIN chewable tablets?
- •
- VETMEDIN capsules come in a different form than VETMEDIN chewable tablets. Unlike VETMEDIN chewable tablets VETMEDIN capsules cannot be cut or divided.
- •
- If your dog has been getting a dose of VETMEDIN chewable tablets that includes cutting a tablet in half, you will need to use a combination of different strengths of VETMEDIN capsules to maintain the same dose.
- •
- Strength - VETMEDIN capsules are not available in 10 mg strength. The number and strength of VETMEDIN capsules administered may be different than for VETMEDIN chewable tablets. For example, a dog that used to receive 10 mg VETMEDIN chewable tablets should receive two 5 mg VETMEDIN capsules instead.
How do I give VETMEDIN capsules to my dog?
VETMEDIN capsules should be given to your dog in their mouth (orally) twice a day, about 12 hours apart as directed by your veterinarian. VETMEDIN capsules should be given about 1 hour before feeding. If you have any questions about administration, please contact your veterinarian.
If your dog vomits after being given VETMEDIN capsules, please contact your veterinarian and unless directed otherwise, do not give additional VETMEDIN capsules again until the next scheduled dose.
What are some of the possible side effects of VETMEDIN capsules?
VETMEDIN capsules may cause side effects similar to those seen with VETMEDIN chewable tablets, even at the prescribed dose. Contact your veterinarian immediately if your dog develops a serious or concerning medical problem or side effect while taking VETMEDIN capsules.
The most common side effects of pimobendan containing products are:
- •
- poor appetite
- •
- lethargy
- •
- diarrhea
- •
- difficulty breathing or coughing
- •
- weakness or difficulty walking
- •
- a rise in heart rate
- •
- vomiting
There are other side effects which may occur with either VETMEDIN capsules or VETMEDIN chewable tablets. For a complete list, ask your veterinarian.
To report a suspected adverse drug event (side effect) or a product quality problem contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251. Adverse drug events and product quality problems may also be reported directly to FDA by completing the online form available athttp://www.fda.gov/reportanimalaeor by requesting a hard copy of the form at 1-888-FDA-VETS.
What if my dog receives more VETMEDIN capsules than what is prescribed?
Contact your veterinarian immediately.
What else should I know about VETMEDIN capsules?
VETMEDIN capsules are not for use in humans.
You should keep VETMEDIN capsules in a secure storage area out of the reach of children, as well as dogs, cats and other animals to prevent accidental ingestion or overdose.
If VETMEDIN capsules are accidentally ingested by a person, contact a physician. It is important to show the treating physician a copy of the VETMEDIN capsules package insert, label, or this information sheet. VETMEDIN capsules are non-sympathomimetic, non-glycoside inotropic drug with vasodilatative properties.
This information sheet contains a summary of important information about VETMEDIN capsules. For more detailed information about VETMEDIN capsules, talk with your veterinarian.
- Storage Statement: VETMEDIN capsules should be stored at temperatures below 25°C (77°F) in a tightly closed container.
Updated 02/2021
Description
The active ingredient in Vetmedin® is pimobendan.
Vetmedin® 1.25 mg capsule contains 1.25 mg pimobendan.
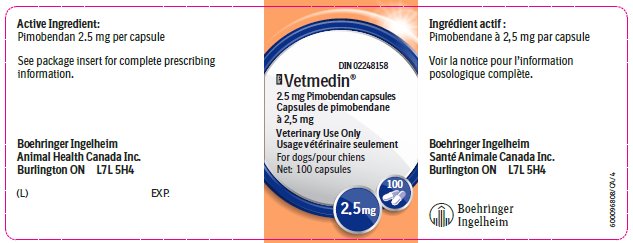
Vetmedin® 2.5 mg capsule contains 2.5 mg pimobendan.
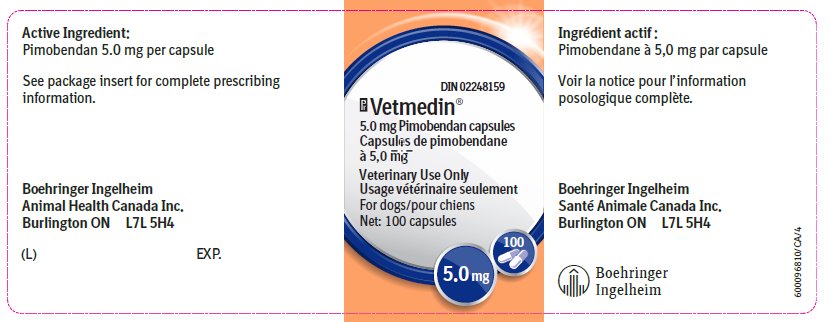
Vetmedin® 5.0 mg capsule contains 5.0 mg pimobendan.
Pimobendan, a benzimidazole-pyridazinone derivative, is a nonsympathomimetic, non-glycoside inotropic drug with vasodilatory properties.
Therapeutic Class
Positive inotropic vasodilator
Indications: Dogs
1. For the treatment of congestive heart failure originating from dilated cardiomyopathy or valvular insufficiency. It is recommended that the diagnosis of congestive heart failure be confirmed by radiographs or diuretic responsiveness.
Treatment should be initiated only in symptomatic cases which will benefit from increased myocardial contractility (positive inotropy).
2. To delay the time of onset of congestive heart failure or sudden death in Doberman Pinscher dogs with clinically asymptomatic dilated cardiomyopathy.
Clinically asymptomatic dilated cardiomyopathy is characterized by an increase in left ventricular end-systolic and end-diastolic diameter and should be diagnosed by means of a comprehensive cardiac examination (including echocardiographic examination and possibly Holter monitoring).
Dosage and Administration
Vetmedin® capsules should be administered orally at a dose range of 0.2 mg to 0.6 mg pimobendan/kg body weight per day. The preferable daily dose is 0.5 mg pimobendan/kg body weight. The dose should be divided into two administrations (0.25 mg/kg each), one half of the dose in the morning and the other half approximately 12 hours later. Each dose should be given approximately one hour before feeding.
Vetmedin® capsules may be combined with a diuretic treatment such as furosemide in dogs with pulmonary edema and/or ascites associated with congestive heart failure.
Contraindications
Vetmedin® capsules should not be used in cases of hypertrophic cardiomyopathies or clinical conditions where an augmentation of cardiac output is not possible for functional or anatomical reasons (e.g. aortic stenosis).
Caution
Cardiac arrhythmias may indicate a more guarded prognosis. According to good veterinary practice, dogs with congestive heart failure should be monitored for presence of arrhythmias during cardiac therapy. Appropriate anti-arrhythmic should be initiated if indicated. The safety in pregnant and lactating dogs has not been established. In studies with rats and rabbits, pimobendan had no effect on fertility and embryotoxic effects only occurred in maternotoxic doses. In rat experiments it has been shown that pimobendan is excreted into milk. Therefore, Vetmedin® capsules should only be administered to pregnant and lactating bitches if the expected therapeutic benefits overweigh the potential risk.
Warning
Warning: KEEP OUT OF REACH OF CHILDREN.
If poisoning occurs, contact a doctor or Poisons Information Center.
Adverse Reaction
Pimobendan administered as an overdose orally may result in profuse vomiting. Patients should be treated symptomatically.
The following suspected adverse effects have been reported following clinical use.
Cardiovascular: Tachycardia (may be dose dependent and avoided by reducing the dose).
Gastrointestinal: Vomiting, diarrhea, inappetence.
Nervous system/Behavioural: Uneasiness, incoordination, convulsions.
Renal: Polyuria, polydypsia.
Clinical Pharmacology
Pimobendan exerts its stimulatory myocardial effect by a dual mechanism of action: increase in calcium sensitivity of cardiac myofilaments and inhibition of phosphodiesterase (type III). It also exhibits a vasodilating action through an inhibitory action on phosphodiesterase III activity.
Following oral administration of Vetmedin® capsules, the absolute bioavailability of the active principle is 60–63%. Mean plasma protein binding is 93%. The plasma elimination half-life of pimobendan is approximately 30 minutes and the main active metabolite elimination half-life is approximately 2 hours. Almost the entire dose is eliminated via feces.
Drug Interactions
There is always a risk of drug interactions when using multiple medications in a compromised or geriatric patient. Use with caution with other positive inotropes. As pimobendan is highly protein-bound, monitor carefully if using other drugs with high protein binding. Concurrent use of beta- blockers or calcium- channel blockers may decrease pimobendan-induced effects on myocardial contractility.
Safety and Efficacy Study Information
The tolerance of pimobendan has been evaluated in pre-clinical studies in healthy Beagle dogs with non-diseased hearts. Daily intravenous administration of pimobendan at dosages of 0.5 mg/kg to 8.0 mg/kg over a period of 2 to 4 weeks was associated with exaggerated myocardial contractility and jet lesions to the myocardium. These intravenous dosages are equivalent to one-time oral dosages of 0.8 mg/kg to 12.8 mg/kg. Lesions were not seen at intravenous dosages of 0.25 mg/kg (equivalent to 0.4 mg/kg orally) administered over a 2 to 4 week period.
In a randomized, blinded placebo controlled study, 76 client-owned Doberman Pinschers were recruited at 10 centers in the UK, USA and Canada. The dogs had preclinical dilated cardiomyopathy (asymptomatic with an increase in left ventricular end-systolic and end-diastolic diameter following echocardiographic diagnosis). Dogs were allocated in a 1:1 ratio to receive pimobendan or a visually identical placebo. The primary endpoint was the time to onset of overt (clinical) dilated cardiomyopathy (DCM) defined as congestive heart failure or sudden death. The time to the onset of CHF or sudden death was statistically significantly improved in the pimobendan treated dogs compared with the placebo treated dogs (hazard ratio [HR] 0.319; 95% CI: 0.167 to 0.617; log rank test p=0.0088), corresponding to a 68.1% reduction in the risk of CHF or sudden death and an increase in the median time to onset of CHF or sudden death to 718 days for the pimobendan from 441 days for placebo. Additionally, in the first 20 to 56 days of up to a 5 year study dogs treated with pimobendan in the preclinical stage of dilated cardiomyopathy the median change (range) in LVIDS was -4 mm (-11.7 to 3mm) and LVIDD was -3.1 mm (-11.7 to 4mm). In the placebo treated dogs the median change in LVIDS was 0 mm (-7.8 to 5.5mm) and LVIDD was 0.8 mm (-6.6 to 6mm).
Boehringer Ingelheim Animal Health Canada Inc.
Burlington ON L7L 5H4
Revised: 2020-09
Vetmedin® is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under license.
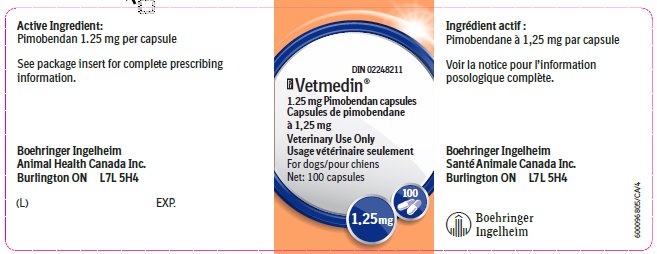
Principal Display Panel – 1.25 mg bottle label, 100 capsules
DIN 02248211
Vetmedin®
1.25 mg Pimobendan capsules
Veterinary Use Only
For dogs
Net: 100 capsules
Principal Display Panel – 1.25 mg display carton, 100 capsules
DIN 02248211
Vetmedin®
1.25 mg Pimobendan capsules
Veterinary Use Only
For dogs
Net Contents: 100 capsules
| VETMEDIN
pimobendan capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VETMEDIN
pimobendan capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VETMEDIN
pimobendan capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091) |