FULL PRESCRIBING INFORMATION
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs are contraindicated in women who are over 35 years of age and smoke [see Contraindications ( 4)] .
1 INDICATIONS AND USAGE
Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets are indicated for use by females of reproductive potential to prevent pregnancy.
2 DOSAGE AND ADMINISTRATION
2.1 How to Start Levonorgestrel and Ethinyl Estradiol Tablets, USP and Ferrous Bisglycinate Tablets
Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets are dispensed in a blister card [see How Supplied/Storage and Handling (16)] . Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets may be started using either a Day 1 start or a Sunday start (see Table 1). For the first cycle of a Sunday Start regimen, an additional method of contraception should be used until after the first 7 consecutive days of administration.
2.2 How to Take Levonorgestrel and Ethinyl Estradiol Tablets, USP and Ferrous Bisglycinate Tablets
Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets (orange active tablets and blue placebo tablets) are swallowed whole once a day
| Complete instructions to facilitate patient counseling on proper tablet usage are located in the FDA-Approved Patient Labeling. | |
|
Starting COCs in women not currently using hormonal contraception (Day 1 Start or Sunday Start) Important: Consider the possibility of ovulation and conception prior to initiation of this product. Tablet Color:
|
Day 1 Start:
Sunday Start:
|
|
Switching to levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets from another hormonal contraceptive |
Start on the same day that a new pack of the previous hormonal contraceptive would have started. |
|
Switching from another contraceptive method to levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets |
Start levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets: |
|
|
|
|
|
|
|
|
|
|
Starting Levonorgestrel and Ethinyl Estradiol Tablets, USP and Ferrous Bisglycinate Tablets after Abortion or Miscarriage
First-trimester
- After a first-trimester abortion or miscarriage, levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets may be started immediately. An additional method of contraception is not needed if levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets are started within 5 days after termination of the pregnancy.
- If levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets are not started within 5 days after termination of the pregnancy, the patient should use additional non-hormonal contraception (such as condoms or spermicide) for the first seven days of her first cycle pack of levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets.
Second-trimester
- Do not start until 4 weeks after a second-trimester abortion or miscarriage, due to the increased risk of thromboembolic disease. Start levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets following the instructions in Table 1 for Day 1 or Sunday start, as desired. If using Sunday start, use additional non-hormonal contraception (such as condoms or spermicide) for the first seven days of the patient’s first cycle pack of levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets. [See Contraindications ( 4), Warnings and Precautions ( 5.1), and FDA-Approved Patient Labeling.]
Starting Levonorgestrel and Ethinyl Estradiol Tablets, USP and Ferrous Bisglycinate Tablets after Childbirth
- Do not start until 4 weeks after delivery, due to the increased risk of thromboembolic disease. Start contraceptive therapy with levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets following the instructions in Table 1 for women not currently using hormonal contraception.
- If the woman has not yet had a period postpartum, consider the possibility of ovulation and conception occurring prior to use of levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets.
[See Contraindications ( 4), Warnings and Precautions ( 5.1), Use in Specific Populations ( 8.1and 8.2), and FDA-Approved Patient Labeling] .
2.3 Missed Tablets
|
Take the tablet as soon as possible. Continue taking one tablet a day until the pack is finished. |
|
Take the two missed tablets as soon as possible and the next two active tablets the next day. Continue taking one tablet a day until the pack is finished. Additional non-hormonal contraception (such as condoms or spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. |
|
Day 1 start:Throw out the rest of pack and start a new pack that same day. Sunday start:Continue taking one tablet a day until Sunday, then throw out the rest of the pack and start a new pack that same day. Additional non-hormonal contraception (such as condoms or spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. |
2.4 Advice in Case of Gastrointestinal Disturbances
In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting or diarrhea occurs within 3 to 4 hours after taking an active tablet, handle this as a missed tablet [see FDA-Approved Patient Labeling] .
3 DOSAGE FORMS AND STRENGTHS
Levonorgestrel and ethinyl estradiol tablets, USP, and ferrous bisglycinate tablets are available in a 28-tablet compact blister card with:
- 21 orange, round biconvex tablets (active) debossed with “A3” on one side and each containing levonorgestrel 0.10 mg and ethinyl estradiol 0.02 mg
- 7 blue, round biconvex tablets (inactive placebo) debossed with “F1” on one side and each containing ferrous bisglycinate 36.5 mg
The ferrous bisglycinate tablets do not serve any therapeutic purpose.
4 CONTRAINDICATIONS
Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets are contraindicated in females who are known to have or develop the following conditions:
- A high risk of arterial or venous thrombotic disease. Examples include women who are known to:
- Smoke, if over age 35 [see Boxed Warning and Warnings and Precautions (5.1)] .
- Have deep vein thrombosis or pulmonary embolism, now or in the past [see Warnings and Precautions (5.1)] .
- Have inherited or acquired hypercoagulopathies [see Warnings and Precautions (5.1)] .
- Have cerebrovascular disease [see Warnings and Precautions (5.1)] .
- Have coronary artery disease [see Warnings and Precautions (5.1)] .
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions (5.1)] .
- Have uncontrolled hypertension [see Warnings and Precautions (5.4)] .
- Have diabetes mellitus with vascular disease [see Warnings and Precautions (5.6)] .
- Have headaches with focal neurological symptoms or have migraine headaches with aura [see Warnings and Precautions (5.7)] .
- Women over age 35 with any migraine headaches [see Warnings and Precautions (5.7)] .
- Liver tumors, benign or malignant, or liver disease [see Warnings and Precautions (5.2)].
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.8)] .
- Pregnancy, because there is no reason to use COCs during pregnancy [see Warnings and Precautions (5.10) and Use in Specific Populations (8.1)] .
- Current diagnosis of, or history of, breast cancer, which may be hormone-sensitive [see Warnings and Precautions (5.11)] .
- Hypersensitivity to any of the components.
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see Warnings and Precautions (5.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Thrombotic Disorders and Other Vascular Problems
- Stop levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if an arterial thrombotic event or venous thromboembolic (VTE) event occurs.
- Stop levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
- If feasible, stop levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of VTE as well as during the following prolonged immobilization.
- Start levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum VTE decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
- The use of COCs increases the risk of VTE. However, pregnancy increases the risk of VTE as much or more than the use of COCs. The risk of VTE in women using COCs is 3 to 9 per 10,000 woman-years. The risk of VTE is highest during the first year of use of COCs and when restarting hormonal contraception after a break of 4 weeks or longer. The risk of thromboembolic disease due to COCs gradually disappears after use is discontinued.
- Use of COCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events. COCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes). The risk increases with age, particularly in women over 35 years of age who smoke.
- Use COCs with caution in women with cardiovascular disease risk factors.
5.2 Liver Disease
Impaired Liver Function
Do not use levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets in women with liver disease, such as acute viral hepatitis or severe (decompensated) cirrhosis of liver [see Contraindications ( 4)] . Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded. Discontinue levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if jaundice develops.
Liver Tumors
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets are contraindicated in women with benign and malignant liver tumors [see Contraindications ( 4)] . Hepatic adenomas are associated with COC use. An estimate of the attributable risk is 3.3 cases/100,000 COC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (>8 years) COC users. However, the risk of liver cancers in COC users is less than one case per million users.
5.3 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as COCs. Discontinue levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications ( 4)] .
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
5.4 High Blood Pressure
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets are contraindicated in women with uncontrolled hypertension or hypertension with vascular disease [see Contraindications ( 4)] . For women with well-controlled hypertension, monitor blood pressure and stop levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if blood pressure rises significantly.
An increase in blood pressure has been reported in women taking COCs, and this increase is more likely in older women with extended duration of use. The incidence of hypertension increases with increasing concentrations of progestin.
5.5 Gallbladder Disease
Studies suggest a small increased relative risk of developing gallbladder disease among COC users. Use of COCs may worsen existing gallbladder disease. A past history of COC-related cholestasis predicts an increased risk with subsequent COC use. Women with a history of pregnancy-related cholestasis may be at an increased risk for COC related cholestasis.
5.6 Carbohydrate and Lipid Metabolic Effects
Carefully monitor prediabetic and diabetic women who take levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets. COCs may decrease glucose tolerance.
Consider alternative contraception for women with uncontrolled dyslipidemia. A small proportion of women will have adverse lipid changes while on COCs.
Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
5.7 Headache
If a woman taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if indicated.
Consider discontinuation of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets in the case of increased frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event).
5.8 Bleeding Irregularities and Amenorrhea
Unscheduled Bleeding and Spotting
Unscheduled (breakthrough or intracyclic) bleeding and spotting sometimes occur in patients on COCs, especially during the first three months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. If pathology and pregnancy are excluded, bleeding irregularities may resolve over time or with a change to a different contraceptive product.
In the clinical trial with levonorgestrel 0.1 mg and ethinyl estradiol 0.02 mg tablets breakthrough bleeding and spotting was reported in 4% and 12% of cycles, respectively. Breakthrough bleeding and spotting occurred together during 11% of the cycles.
Amenorrhea and Oligomenorrhea
Women who use levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets may experience amenorrhea. In the clinical trial, 2.6% of the evaluable cycles were amenorrheic. Some women may experience amenorrhea or oligomenorrhea after discontinuation of COCs, especially when such a condition was preexistent.
If scheduled (withdrawal) bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
5.9 FD&C Yellow No. 5 Allergic-type Reaction
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
5.10 Depression
Carefully observe women with a history of depression and discontinue levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if depression recurs to a serious degree.
5.11 Malignant Neoplasms
Breast Cancer
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets are contraindicated in females who currently have or have had breast cancer because breast cancer may be hormonally sensitive [see Contraindications (4)].
Epidemiology studies have not found a consistent association between use of combined oral contraceptives (COCs) and breast cancer risk. Studies do not show an association between ever (current or past) use of COCs and risk of breast cancer. However, some studies report a small increase in the risk of breast cancer among current or recent users (<6 months since last use) and current users with longer duration of COC use [see Postmarketing Experience (6.2)].
Cervical Cancer
Some studies suggest that COC use has been associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
5.12 Effect on Binding Globulins
The estrogen component of COCs may raise the serum concentrations of thyroxine-binding globulin, sex hormone-binding globulin, and cortisol-binding globulin. The dose of replacement thyroid hormone or cortisol therapy may need to be increased.
5.13 Monitoring
A woman who is taking COCs should have her blood pressure checked periodically with her healthcare provider.
6 ADVERSE REACTIONS
The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
- Serious cardiovascular events and stroke [see Boxed Warning and Warnings and Precautions ( 5.1)]
- Vascular events [see Warnings and Precautions ( 5.1)]
- Liver disease [see Warnings and Precautions ( 5.2)]
Adverse reactions commonly reported by COC users are:
- Irregular uterine bleeding
- Nausea
- Breast tenderness
- Headache
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In a clinical trial with levonorgestrel 0.1 mg and ethinyl estradiol 0.02 mg tablets, a total of 1477 healthy women of child-bearing potential were enrolled and had 7870 cycles of exposure. Of these, 792 subjects had completed 6 cycles of treatment. The women ranged in age from 17 to 49 years and 87% were Caucasian.
Common Adverse Reactions (≥ 2% of women):
- headache (14%)
- metrorrhagia (8%)
- dysmenorrhea (7%)
- nausea (7%)
- abdominal pain (4%)
- breast pain (4%)
- emotional lability (3%)
- acne (3%)
- depression (2%)
- amenorrhea (2%)
- vaginal moniliasis (2%)
At the time of the report, 133 (9%) subjects had withdrawn from the study due to adverse events. The most frequent were due to headache and metrorrhagia (1% each). Other adverse events occurring in < 1% of those who discontinued included amenorrhea, depression, emotional lability, hypertension, acne, menorrhagia, nausea, hypercholesterolemia, weight gain, dysmenorrhea, and flatulence. All other reasons for discontinuation were reported by 3 or fewer subjects.
6.2 Postmarketing Experience
Five studies that compared breast cancer risk between ever-users (current or past use) of COCs and never-users of COCs reported no association between ever use of COCs and breast cancer risk, with effect estimates ranging from 0.90 - 1.12 (Figure 1).
Three studies compared breast cancer risk between current or recent COC users (<6 months since last use) and never users of COCs (Figure 1). One of these studies reported no association between breast cancer risk and COC use. The other two studies found an increased relative risk of 1.19 - 1.33 with current or recent use. Both of these studies found an increased risk of breast cancer with current use of longer duration, with relative risks ranging from 1.03 with less than one year of COC use to approximately 1.4 with more than 8-10 years of COC use.
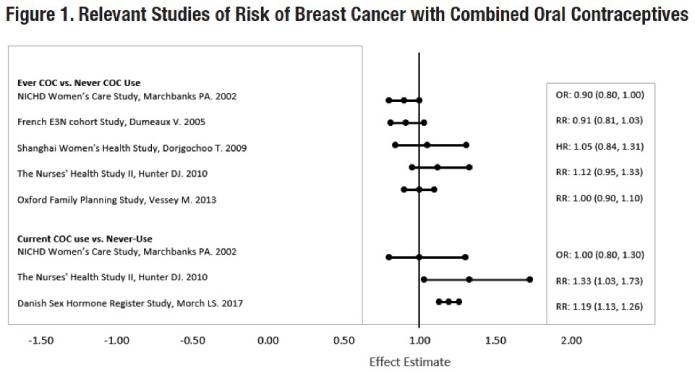
RR = relative risk; OR = odds ratio; HR = hazard ratio. “ever COC” are females with current or past COC use; “never COC use” are females that never used COCs.
The following additional adverse drug reactions have been reported from worldwide postmarketing experience with levonorgestrel 0.1 mg and ethinyl estradiol 0.02 mg tablets.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorder: chest pain, dyspnea, palpitations
Gastrointestinal disorders: abdominal pain, nausea, vomiting, diarrhea
General disorders and administration site conditions: chest pain, fatigue, pain, malaise, injection site pain or erythema, feeling abnormal, pyrexia, condition aggravated, asthenia
Immune system disorders: hypersensitivity reactions, including pruritus, rash, urticaria,erythema
Injury, poisoning, and procedural complications: injury
Investigations: weight decreased
Musculoskeletal and connective tissue disorders: pain in extremity, arthralgia, back pain,muscle spasm
Nervous system disorders: headache, migraine, dizziness, hypoesthesia, paresthesia
Psychiatric disorders: depression, insomnia, anxiety
Reproductive system and breast disorders: metrorrhagia, menorrhagia, hot flush, vaginal hemorrhage
Respiratory, thoracic, and mediastinal disorders: nasopharyngitis, cough
Sleep disorders and disturbances: somnolence
Vascular disorders: deep vein thrombosis, pulmonary embolism
7 DRUG INTERACTIONS
Consult the labeling of concurrently used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
7.1 Effects of Other Drugs on Combined Oral Contraceptives
Substances decreasing the plasma concentrations of COCs and potentially diminishing the efficacy of COCs:
Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the plasma concentrations of COCs and potentially diminish the effectiveness of COCs or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, topiramate, rifabutin, rufinamide, aprepitant, and products containing St. John’s wort. Interactions between hormonal contraceptives and other drugs may lead to breakthrough bleeding and/or contraceptive failure. Counsel women to use an alternative method of contraception or a back-up method when enzyme inducers are used with COCs, and to continue back-up contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Colesevelam: Colesevelam, a bile acid sequestrant, given together with a COC, has been shown to significantly decrease the AUC of ethinyl estradiol (EE). The drug interaction between the contraceptive and colesevelam was decreased when the two drug products were given 4 hours apart.
Substances increasing the plasma concentrations of COCs:
Co-administration of atorvastatin or rosuvastatin and certain COCs containing EE increase AUC values for EE by approximately 20-25%. Ascorbic acid and acetaminophen may increase plasma EE concentrations, possibly by inhibition of conjugation. CYP3A4 inhibitors, such as itraconazole, voriconazole, fluconazole, grapefruit juice, or ketoconazole may increase plasma hormone concentrations.
Human immunodeficiency virus (HIV)/ Hepatitis C virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors:
Significant changes (increase or decrease) in the plasma concentrations of estrogen and/or progestin have been noted in some cases of co-administration with HIV/HCV protease inhibitors and non-nucleoside reverse transcriptase inhibitors (decrease [e.g., nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, tipranavir/ritonavir, boceprevir, telaprevir, nevirapine and efavirenz] or increase [e.g., indinavir, atazanavir/ritonavir and etravirine]).
7.2 Effects of Combined Oral Contraceptives on Other Drugs
Combined oral contraceptives containing EE may inhibit the metabolism of other compounds (e.g., cyclosporine, prednisolone, theophylline, tizanidine, and voriconazole) and increase their plasma concentrations. Combined oral contraceptives have been shown to decrease plasma concentrations of acetaminophen, clofibric acid, morphine, salicylic acid, temazepam and lamotrigine. Significant decrease in plasma concentration of lamotrigine has been shown, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary.
Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because the serum concentration of thyroid-binding globulin increases with use of COCs [see Warnings and Precautions ( 5.12)] .
7.3 Concomitant Use with HCV Combination Therapy - Liver Enzyme Elevation
Do not co-administer levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets with HCV drug combinations containing ombitasvir/ paritaprevir/ritonavir, with or without dasabuvir, due to potential for ALT elevations [see Warnings and Precautions ( 5.3)] .
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets are contraindicated in pregnancy because there is no reason to use combined hormonal contraceptives (CHCs) in pregnancy. Discontinue levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if pregnancy occurs. Based on epidemiologic studies and meta-analyses, there is little or no increased risk of birth defects in the children of females who inadvertently use COCs during early pregnancy (See Data) .
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
8.2 Lactation
Risk Summary
Combined hormonal contraceptives (CHCs) and/or metabolites are present in human milk and in breast-fed infants. CHCs, including levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets, can reduce milk production in breast-feeding females. This reduction can occur at any time but is less likely to occur once breast-feeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breast-feeding. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets and any potential adverse effects on the breast-fed child from levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets or from the underlying maternal condition.
8.4 Pediatric Use
Safety and efficacy of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets have been established in women of reproductive age. Efficacy is expected to be the same in post-pubertal adolescents under the age of 18 years as for users 18 years and older. Use of this product before menarche is not indicated.
8.5 Geriatric Use
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets have not been studied in postmenopausal women and is not indicated in this population.
8.6 Hepatic Impairment
The pharmacokinetics of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets have not been studied in women with hepatic impairment. However, steroid hormones may be poorly metabolized in patients with hepatic impairment. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded [see Contraindications ( 4) and Warnings and Precautions ( 5.2)] .
10 OVERDOSAGE
There have been no reports of serious ill effects from overdose of oral contraceptives, including ingestion by children. Overdosage may cause withdrawal bleeding in females and nausea.
11 DESCRIPTION
Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets provide an oral contraceptive regimen consisting of 21 orange active tablets and 7 blue inactive tablets.
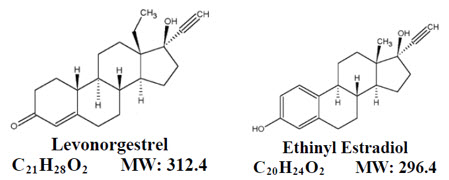
- 21 orange active tablets each containing 0.10 mg of levonorgestrel, d(-)-13β-ethyl-17α-ethinyl-17β-hydroxygon-4-en-3-one, a totally synthetic progestogen, and 0.02 mg of ethinyl estradiol, 17α-ethinyl-1,3,5(10)-estratriene-3, 17β-diol, an estrogenic compound.
- 7 blue inactive tablets each containing 36.5 mg ferrous bisglycinate.
The inactive ingredients present in orange active tablet are FD&C Yellow #5 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, FD&C Red #40 Aluminum Lake, titanium dioxide, polyvinyl alcohol, talc, macrogol/polyethylene glycol 3350 NF, lecithin (soya), iron oxide black, lactose monohydrate, magnesium stearate and pregelatinized starch.
Each inactive blue tablet contains the following inactive ingredients: ferrous bisglycinate, citric acid, glycine, maltodextrin , silica, microcrystalline cellulose NF, magnesium stearate NF, croscarmellose sodium NF, colloidal silicon dioxide NF, hypromellose type 2910, titanium dioxide, polyethylene glycol 400, FD&C Red #40 Aluminum Lake, FD&C Yellow #6 Aluminum Lake and FD&C Blue #1 Aluminum Lake.
Levonorgestrel has the empirical formula of C 21H 28O 2and the molecular weight of 312.4, and ethinyl estradiol has the empirical formula of C 20H 24O 2and the molecular weight of 296.4.
The molecular structures are provided below:
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
COCs lower the risk of becoming pregnant primarily by suppressing ovulation. Other possible mechanisms may include cervical mucus changes that inhibit sperm penetration and endometrial changes that reduce the likelihood of implantation.
12.2 Pharmacodynamics
No specific pharmacodynamics studies were conducted with levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
12.3 Pharmacokinetics
Absorption
No specific investigation of the absolute bioavailability of levonorgestrel and ethinyl estradiol tablets USP in humans has been conducted. However, literature indicates that levonorgestrel is rapidly and completely absorbed after oral administration (bioavailability about 100%) and is not subject to first-pass metabolism. Ethinyl estradiol is rapidly and almost completely absorbed from the gastrointestinal tract but, due to first-pass metabolism in gut mucosa and liver, the bioavailability of ethinyl estradiol is between 38% and 48%.
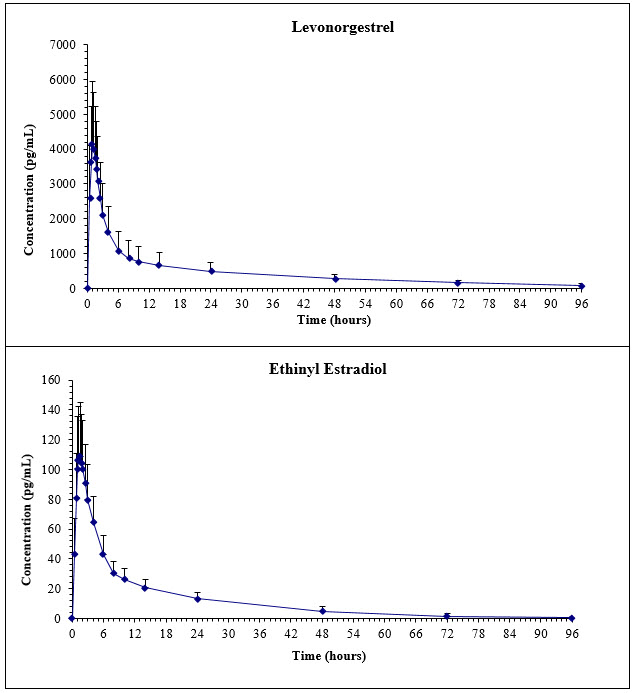
After a single dose of two levonorgestrel and ethinyl estradiol tablets to 34 women under fasting conditions, the mean (± SD) plasma area under the concentration time curve (AUC) and maximum concentration (Cmax) of levonorgestrel were 41.7 ± 18.0 ng*hour/mL and 4.4 ± 1.8ng/mL, respectively, with a median time to maximum concentration (Tmax) of 1.0 hours. The mean (±SD) plasma AUC and Cmax of ethinyl estradiol were 1167 ± 367 pg*hour/mL and 115 ± 37 pg/mL, respectively, with a median Tmax of 1.5 hours. The plasma levonorgestrel and ethinyl estradiol pharmacokinetic profi les following a single dose of two levonorgestrel and ethinyl estradiol tablets are shown in Figure 2.
Figure 2. Mean (SD) Levonorgestrel and Ethinyl Estradiol Plasma Concentrations in 34 Subjects receiving two Levonorgestrel and Ethinyl Estradiol Tablets (0.1 mg/0.02 mg) from Levonorgestrel and Ethinyl Estradiol Tablets and Ferrous Bisglycinate Tablets
Distribution
Levonorgestrel in serum is primarily bound to SHBG. Ethinyl estradiol is about 97% bound to plasma albumin. Ethinyl estradiol does not bind to SHBG, but induces SHBG synthesis.
Metabolism
Levonorgestrel:The most important metabolic pathway occurs in the reduction of the Δ4-3-oxo group and hydroxylation at positions 2α, 1β, and 16β, followed by conjugation. Most of the metabolites that circulate in the blood are sulfates of 3α, 5β-tetrahydro-levonorgestrel, while excretion occurs predominantly in the form of glucuronides. Some of the parent levonorgestrel also circulates as 17β-sulfate. Metabolic clearance rates may differ among individuals by several-fold, and this may account in part for the wide variation observed in levonorgestrel concentrations among users.
Ethinyl estradiol:Cytochrome P450 enzymes (CYP3A4) in the liver are responsible for the 2‑hydroxylation that is the major oxidative reaction. The 2-hydroxy metabolite is further transformed by methylation and glucuronidation prior to urinary and fecal excretion. Levels of Cytochrome P450 (CYP3A) vary widely among individuals and can explain the variation in rates of ethinyl estradiol 2-hydroxylation. Ethinyl estradiol is excreted in the urine and feces as glucuronide and sulfate conjugates, and undergoes enterohepatic circulation.
Excretion
The elimination half-life for levonorgestrel is approximately 34 ± 14 hours following a single dose. Levonorgestrel and its metabolites are primarily excreted in the urine (40% to 68%) and about 16% to 48% are excreted in feces. The elimination half-life of ethinyl estradiol is 17 ± 5.7 hours.
14 CLINICAL STUDIES
In a clinical trial with levonorgestrel 0.1 mg and ethinyl estradiol 0.02 mg tablets, 1,477 women aged 17-49 years, had 7,720 cycles of use. Eighty-seven percent (87%) of the women were Caucasian. The average weight was 66.4 kg with a range of 38.0-154.2 kg. Among the women in the trial, 5.3% had never used COCs.
A total of 5 pregnancies were reported. This represents an overall pregnancy rate of approximately 1 pregnancy per 100 woman-years.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets are available in a blister pack containing 28 tablets arranged in 3 rows of 7 active tablets and 1 row of inactive tablets, as follows:
- 21 active tablets: orange, round tablet debossed with “A3” on one side; each tablet containing levonorgestrel 0.10 mg and ethinyl estradiol 0.02 mg
- 7 inactive tablets: blue, round tablet debossed with “F1” on one side; each tablet containing ferrous bisglycinate 36.5 mg
Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets are available in the following configurations:
Carton of one 1-cycle blister pack (NDC 42192-623-28)
Carton of three 1-cycle blister packs (NDC 42192-623-03)
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling(Patient Information and Instructions for Use).
Counsel patients on the following information:
- Cigarette smoking increases the risk of serious cardiovascular events from COC use, and that women who are over 35 years old and smoke should not use COCs [see Boxed Warning] .
- Increased risk of VTE compared to non-users of COCs is greatest after initially starting a COC or restarting (following a 4-week or greater pill-free interval) the same or a different COC [see Warnings and Precautions ( 5.1)] .
- Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets do not protect against HIV-infection (AIDS) and other sexually transmitted diseases.
- Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets are not to be used during pregnancy; if pregnancy occurs during use of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets, instruct the patient to stop further use [see Use in Specific Populations ( 8.1)] .
- Take one tablet daily by mouth at the same time every day. Instruct patients what to do in the event pills are missed [see Dosage and Administration ( 2.3)] .
- Use a back-up or alternative method of contraception when enzyme inducers are used with levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets [see Drug Interactions ( 7.1)] .
- COCs may reduce breast milk production; this is less likely to occur if breastfeeding is well established [see Use in Specific Populations ( 8.2)] .
- A woman who starts COCs postpartum and who has not yet had a period should use an additional method of contraception until she has taken a white tablet for 7 consecutive days [see Dosage and Administration ( 2.2)] .
- Amenorrhea may occur. Consider pregnancy in the event of amenorrhea at the time of the first missed period. Rule out pregnancy in the event of amenorrhea in two or more consecutive cycles [see Warnings and Precautions ( 5.8)] .

Manufactured for:
Acella Pharmaceuticals, LLC.,
1880 McFarland Pkwy.,
Alpharetta, GA 30005
1-800-541-4802
Manufactured by:
Novast Laboratories, Ltd.,
Nantong, China 226009.
Date: 08/2022
I0162 Rev. B
PATIENT INFORMATION
Levonorgestrel and Ethinyl Estradiol (LEE-voe-nor-JES-trel and EH-thih-nill-ess-tra-DYE-ole) Tablets, USP and Ferrous Bisglycinate (FARR-ouss BISS-glyy-sinn-ATE) Tablets
for oral administration
What is the most important information I should know about levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
Do not use levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects (heart and blood vessel problems) from birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
What are levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets are birth control pills (oral contraceptive) used by women to prevent pregnancy.
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets do not protect against HIV infections (AIDS) and other sexually transmitted infections.
How do levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets work for contraception?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The better you follow the directions, the less chance you have of getting pregnant.
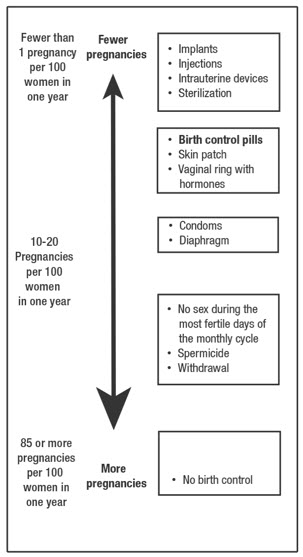
Based on the results of one clinical study of a 28-day regimen of levonorgestrel 0.1 mg/ethinyl estradiol 0.02 mg tablets, about 1 out of 100 women may get pregnant within the first year they use levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
Do not take levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if you:
- smoke and are over 35 years of age
- have or have had blood clots in your arms, legs, lungs, or eyes
- have a problem with your blood that makes it clot more than normal
- have certain heart valve problems or irregular heart beat that increases your risk of having blood clots
- had a stroke
- had a heart attack
- have high blood pressure that cannot be controlled by medicine
- have diabetes with kidney, eye, nerve, or blood vessel damage
- have certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision, or any migraine headaches if you are over 35 years of age
- have liver problems, including liver tumors
- have any unexplained vaginal bleeding
- are pregnant
- have or have had breast cancer or any cancer that is sensitive to female hormones
- are allergic to levonorgestrel, ethinyl estradiol, ferrous bisglycinate or any of the ingredients in levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets. Some people who are allergic to aspirin may also be allergic to FD&C Yellow No. 5 (tartrazine). FD&C Yellow No. 5 (tartrazine) is an ingredient in levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets which also may cause an allergic type reaction such as bronchial asthma. See the end of this Patient Information leaflet for a complete list of ingredients in levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
- take any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood.
If any of these conditions happen while you are taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets, stop taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets right away and talk to your healthcare provider. Use non-hormonal contraception when you stop taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
Before you take levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets, tell your health care provider about all of your medical conditions, including if you:
- are scheduled for surgery. Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets may increase your risk of blood clots after surgery. You should stop using your levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets at least 4 weeks before you have surgery and not restart it until at least 2 weeks after your surgery.
- are pregnant or think you may be pregnant
- are depressed now or have been depressed in the past
- had yellowing of your skin or eyes (jaundice) caused by pregnancy (cholestasis of pregnancy)
- are breastfeeding or plan to breastfeed. Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets may decrease the amount of breast milk you make. A small amount of the hormones in levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets may pass into your breast milk. Talk to your healthcare provider about the best birth control method for you while breastfeeding.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins and herbal supplements.
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets may affect the way other medicines work, and other medicines may affect how well levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets work.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
- Read the detailed Instructions for Use at the end of this Patient Information leaflet about the right way to take your levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
What are the possible serious side effects of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
-
Like pregnancy, levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets may cause serious side effects, including blood clots in your lungs, heart attack, or a stroke that may lead to death. Some other examples of serious blood clots include blood clots in the legs or eyes.
Serious blood clots can happen especially if you smoke, are obese, or are older than 35 years of age. Serious blood clots are more likely to happen when you:- first start taking birth control pills
- restart the same or different birth control pills after not using them for a month or more
Call your healthcare provider or go to a hospital emergency room right away if you have:
-
- leg pain that will not go away
- sudden severe shortness of breath
- sudden change in vision or blindness
- chest pain
- a sudden, severe headache unlike your usual headaches
- weakness or numbness in your arm or leg
- trouble speaking
Other serious side effects include:
-
liver problems, including:
- rare liver tumors
- jaundice (cholestasis), especially if you previously had cholestasis of pregnancy. Call your healthcare provider if you have yellowing of your skin or eyes.
- high blood pressure.You should see your health care provider to check your blood pressure regularly.
- gallbladder problems
- changes in the sugar and fat (cholesterol and triglycerides) levels in your blood
- new or worsening headaches including migraine headaches
- depression
- possible cancer in your breast and cervix
- swelling of your skin especially around your mouth, eyes, and in your throat (angioedema).Call your healthcare provider if you have a swollen face, lips, mouth tongue or throat, which may lead to difficulty swallowing or breathing. Your chance of having angioedema is higher if you have a history of angioedema.
- dark patches of skin around your forehead, nose, cheeks and around your mouth, especially during pregnancy (chloasma).Women who tend to get chloasma should avoid spending a long time in sunlight, tanning booths, and under sun lamps while taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets. Use sunscreen if you have to be in the sunlight.
What are the most common side effects of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
The most common side effects of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets include:
- headache (including migraine)
- irregular vaginal bleeding (including absence of period)
- nausea
- breast tenderness, pain and discomfort
- stomach (abdominal) pain
- pain with your periods (menstrual cycle)
- mood changes, including depression
- acne
- vaginal infections
These are not all the possible side effects of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
What else should I know about taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
- If you are scheduled for any lab tests, tell your healthcare provider you are taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets. Certain blood tests may be affected by levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
How should I store levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
- Store levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets and all medicines out of the reach of children.
- Store away from light.
General information about the safe and effective use of levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets for a condition for which it was not prescribed. Do not give levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets that is written for health professionals.
Do birth control pills cause cancer?
It is not known if hormonal birth control pills cause breast cancer. Some studies, but not all, suggest that there could be a slight increase in the risk of breast cancer among current users with longer duration of use.
If you have breast cancer now, or have had it in the past, do not use hormonal birth control because some breast cancers are sensitive to hormones.
Women who use birth control pills may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners.
What if I want to become pregnant?
You may stop taking the pill whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop taking the pill.
What should I know about my period when taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
Some women may miss a period. Irregular vaginal bleeding or spotting may happen while you are taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets, especially during the first few months of use. This usually is not a serious problem. If the irregular vaginal bleeding or spotting continues or happens again after you have had regular menstrual cycles call your healthcare provider. It is important to continue taking your pills on a regular schedule to prevent a pregnancy.
What if I miss my scheduled period when using levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
Some women miss periods on hormonal birth control, even when they are not pregnant. However, if you go 2 or more months in a row without a period, or you miss your period after a month where you did not use all of your levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets correctly, call your healthcare provider because you may be pregnant. Also call your healthcare provider if you have symptoms of pregnancy such as morning sickness or unusual breast tenderness. Stop taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets if you are pregnant.
What are the ingredients in levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
Active ingredients:Orange tablets: levonorgestrel and ethinyl estradiol.
Inactive ingredients: Orange tablets: FD&C Yellow #5 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, FD&C Red #40 Aluminum Lake, titanium dioxide, polyvinyl alcohol, talc, macrogol/polyethylene glycol 3350 NF, lecithin (soya), iron oxide black, lactose monohydrate, magnesium stearate and pregelatinized starch.
Inactive ingredients:Blue tablets: ferrous bisglycinate, citric acid NF, glycine, maltodextrin NF, silica, microcrystalline cellulose NF, magnesium stearate NF, croscarmellose sodium NF, colloidal silicon dioxide NF, hypromellose type 2910, titanium dioxide, polyethylene glycol 400, FD&C Red #40 Aluminum Lake, FD&C Yellow #6 Aluminum Lake and FD&C Blue #1 Aluminum Lake.
For more information, go to www.acellapharma.com or call 1-800-541-4802.
INSTRUCTIONS FOR USE
Levonorgestrel and Ethinyl Estradiol (LEE-voe-nor-JES-trel and EH-thih-nill-ess-tra-DYE-ole) Tablets, USP and Ferrous Bisglycinate (FARR-ouss BISS-glyy-sinn-ATE) Tablets
for oral administration
Important Information about taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets
- Take 1 pill every day at the same time. Take the pills in the order directed on your blister pack.
Both the orange pills and the blue pills should be swallowed whole. - Do not skip your pills, even if you do not have sex often. If you miss pills (including starting the pack late) you could get pregnant. The more pills you miss, the more likely you are to get pregnant.
- If you have trouble remembering to take levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets, talk to your healthcare provider. When you first start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets, spotting or light bleeding in between your periods may occur. Contact your healthcare provider if this does not go away after a few months.
- You may feel sick to your stomach (nauseous), especially during the first few months of taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets. If you feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If your nausea does not go away, call your healthcare provider.
- Missing pills can also cause spotting or light bleeding, even when you take the missed pills later. On the days you take 2 pills to make up for missed pills (see below), you could also feel a little sick to your stomach.
- Some women miss periods on hormonal birth control, even when they are not pregnant. However, if you miss a period and have not taken levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets according to directions, or miss 2 periods in a row, or feel like you may be pregnant, call your health care provider. If you have a positive pregnancy test, you should stop taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
- If you have vomiting or diarrhea within 3 to 4 hours of taking your pill, take another pill of the same color from your extra blister pack. If you do not have an extra blister pack, take the next pill in your blister pack.
- Continue taking all your remaining pills in order. Start the first pill of your next blister pack the day after finishing your current blister pack. This will be 1 day earlier than originally scheduled. Continue on your new schedule.
- If you have vomiting or diarrhea for more than 1 day, your birth control pills may not work as well. Use an additional birth control method, like condoms or a spermicide, until you check with your healthcare provider.
- Stop taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets at least 4 weeks before you have major surgery and do not restart it until at least 2 weeks after your surgery. Be sure to use other forms of contraception (like condoms or spermicide) during this time period.
Before you start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets
- Decide what time of day you want to take your pill. It is important to take it at the same time every day and in the order as directed on your blister pack.
- Have backup contraception (condoms or spermicide) available and an extra full pack of pills as needed.
When should I start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?
If you start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets and you have not used a hormonal birth control method before:
- There are 2 ways to start taking your birth control pills.
- You can either start on a Sunday (Sunday Start) or
- You can start on the first day (Day 1) of your natural menstrual period (Day 1 Start).
Your healthcare provider should tell you when to start taking your birth control pill.
If you use the Sunday Start, use non-hormonal back-up contraception such as condoms or spermicide for the first 7 days that you take levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets. You do not need back-up contraception if you use the Day 1 Start.
If you start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets and you are switching from another birth control pill:
- Start your new levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets pack on the same day that you would start the next pack of your previous birth control method.
- Do not continue taking the pills from your previous birth control pack.
If you start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets and previously used a vaginal ring or transdermal patch:
- Start using levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets on the day you would have reapplied the next ring or patch.
If you start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets and you are switching from a progestin-only method such as an implant or injection:
- Start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets on the day of removal of your implant or on the day when you would have had your next injection.
If you start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets and you are switching from an intrauterine device or system (IUD or IUS):
- Start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets on the day of removal of your IUD or IUS.
- You do not need back-up contraception if your IUD or IUS is removed on the first day (Day 1) of your period. If your IUD or IUS is removed on any other day, use non-hormonal back-up contraception such as condoms or spermicide for the first 7 days that you take levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
Keep a calendar to track your period:
If this is the first time you are taking birth control pills, read, “ When should I start taking levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets?” above. Follow these instructions for either a Sunday Start or a Day 1 Start.
Sunday Start:
You will use a Sunday Start if your healthcare provider told you to take your first pill on a Sunday. Use non-hormonal back-up contraception such as condoms or spermicide for the first 7 days of the first cycle that you take levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets.
Instructions for using your pill pack
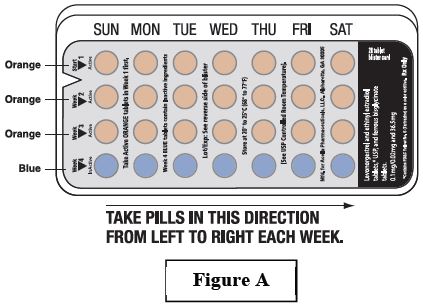
- Look at your levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets pill pack. See Figure A.
- Take pill 1 on the Sunday after your period starts.
- If your period starts on a Sunday, take pill “ 1” that day and refer to Day 1 Start instructions below.
- Take 1 pill every day in the order on the blister pack at the same time each day for 28 days.
- After taking the last pill on Day 28 from the blister pack, start taking the first pill from a new pack, on the same day of the week as the first pack (Sunday). Take the first pill in the new pack whether or not you are having your period.
Day 1 Start:
You will use a Day 1 Start if your healthcare provider told you to take your first pill (Day 1) on the first day of your period.
- Take 1 pill every day in the order of the blister pack, at the same time each day, for 28 days.
- After taking the last pill on Day 28 from the pill dispenser, start taking the first pill from a new pack, on the same day of the week as the first pack. Take the first pill in the new pack whether or not you are having your period.
Instructions for using your pill pack:
Step 1.
Look at your levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets pill pack. See Figure A.
The levonorgestrel and ethinyl estradiol and ferrous bisglycinate pill pack has:
- 21 orange (active) pills with hormone for Week 1 through Week 3.
- 7 blue (inactive) pills without hormones for Week 4
Step 2.
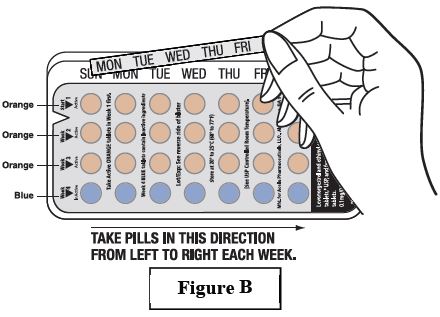
Find what day of the week you are to start taking pills. If your period begins on a day other than Sunday, place the day label strip that starts with the first day of your period. For example, if your period begins on Monday, place the day label strip with Monday as the first day. See Figure B.
Step 3.
Remove the orange pill by pressing the pill through the foil in the bottom of the pill pack. Continue taking the orange pills for 21 days.
Step 4.
On the first day of Week 4 start taking the blue pills. Take the blue pill for 7 days. Your period should start during this time.
Step 5.
When you have taken all of the blue pills in your pill pack, get a new pill pack and start taking the orange pills.
- For a Day 1 start: Begin your next pill pack on the same day of the week as your first cycle pill pack.
- For a Sunday Start: Begin your next pill pack on Sunday.
What should I do if I miss any levonorgestrel and ethinyl estradiol and ferrous bisglycinate pills?
If you miss 1 pill in Weeks 1, 2, or 3, follow these steps:
- Take it as soon as you remember. Take the next pill at your regular time. This means you may take 2 pills in 1 day.
- Then continue taking 1 pill every day until you finish the pack.
- You do not need to use a back-up birth control method if you have sex.
If you miss 2 pills in Week 1 or Week 2 of your pack, follow these steps:
- Take the 2 missed pills as soon as possible and the next 2 pills the next day.
- Then continue to take 1 pill every day until you finish the pack.
- Use a non-hormonal birth control method (such as a condom or spermicide) as a back-up if you have sex during the first 7 days after missing your pills.
If you miss 2 pills in a row in Week 3, or you miss 3 or more pills in a row during Weeks 1, 2, or 3 of the pack, follow these steps:
-
If you are a Day 1 Starter:
- Throw out the rest of the pill pack and start a new pack that same day.
-
If you are a Sunday Starter:
- Keep taking 1 pill every day until Sunday. On Sunday, throw out the rest of the pack and start a new pack of pills that same day.
- You may not have your period this month but this is expected. However, if you miss your period 2 months in a row, call your healthcare provider because you might be pregnant.
- You could become pregnant if you have sex during the first 7 days after you restart your pills. You should use a non-hormonal birth control method (such as a condom or spermicide) as a back-up if you have sex during the first 7 days after you restart your pills.
If you have any questions or are unsure about the information in this leaflet, call your healthcare provider. They have a more technical leaflet called the Professional Labeling which you may wish to read.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Acella Pharmaceuticals, LLC.,
1880 McFarland Pkwy.,
Alpharetta, GA 30005
1-800-541-4802
Manufactured by:
Novast Laboratories, Ltd.,
Nantong, China 226009.
Date: 08/2022 L-0322 Rev 0822-02
Rev. B
PRINCIPAL DISPLAY PANEL - CARTON FRONT

NDC 42192-623-28
28-day regimen
1 Blister Pack
28 tablets
28-day regimen
1 Blister Pack,28 tablets
Rx Only
levonorgestrel and ethinyl estradiol tablets, USP, and ferrous bisglycinate tablets 0.1mg/0.02mg and 36.5mg
This product is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
The inactive ingredients present in orange active tablet are FD&C Yellow #5 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, FD&C Red #40 Aluminum Lake, titanium dioxide, polyvinyl alcohol, talc, macrogol/polyethylene glycol 3350 NF, lecithin (soya), iron oxide black, lactose monohydrate, magnesium stearate and pregelatinized starch.
Each inactive blue tablet contains the following inactive ingredients: ferrous bisglycinate, citric acid, glycine, maltodextrin , silica, microcrystalline cellulose NF, magnesium stearate NF, croscarmellose sodium NF, colloidal silicon dioxide NF, hypromellose type 2910, titanium dioxide, polyethylene glycol 400, FD&C Red #40 Aluminum Lake, FD&C Yellow #6 Aluminum Lake and FD&C Blue #1 Aluminum Lake.
1 Blister Pack, 28 tablets
Levonorgestrel and ethinyl estradiol tablets and ferrous bisglycinate tablets
WARNING:CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS.
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs are contraindicated in women who are over 35 years of age and smoke.
NDC 42192-623-28
28-day regimen
PRINCIPAL DISPLAY PANEL - CARTON BACK
1 Blister Pack,
28 tablets
28-day regimen
1 blister pack,28 tablets
Rx Only
Levonorgestrel and ethinyl estradiol tablets, USP and ferrous bisglycinate tablets 0.1mg/0.02mg and 36.5mg
IMPORTANT - Note to Dispensing Pharmacist:Each compact dispenser contains a combination Patient Information and Instructions For Use Insert with Day Label Stickers. This should be included with each package dispensed to the patient.
Each active orange tablet (21) contains levonorgestrel 0.1 mg and ethinyl estradiol 0.02 mg.
Each inactive blue tablet (7) contains ferrous bisglycinate 36.5 mg.
Contains color additives including FD&C Yellow No. 5 (tartrazine).
Usual Dosage:One orange tablet daily for 21 consecutive days followed by one blue placebo tablet for 7 consecutive days according to prescribed schedule.
See enclosed prescribing information.
Store at 20° to 25°C (68° to 77°F); Excursions permitted to 15° to 30°C (59° to 86°F). [See USP controlled room temperature.]
Protect from light.
Keep out of the reach of children.
WARNING:Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
Manufactured for:
Avion Pharmaceuticals, LLC., Alpharetta, GA 30005
1-800-541-4802
Manufactured by:
Novast Laboratories, Ltd., Nantong, China 226009
Rx Only
L-0319 Rev 0721-01 Iss. 07/2021 Rev A C0248
