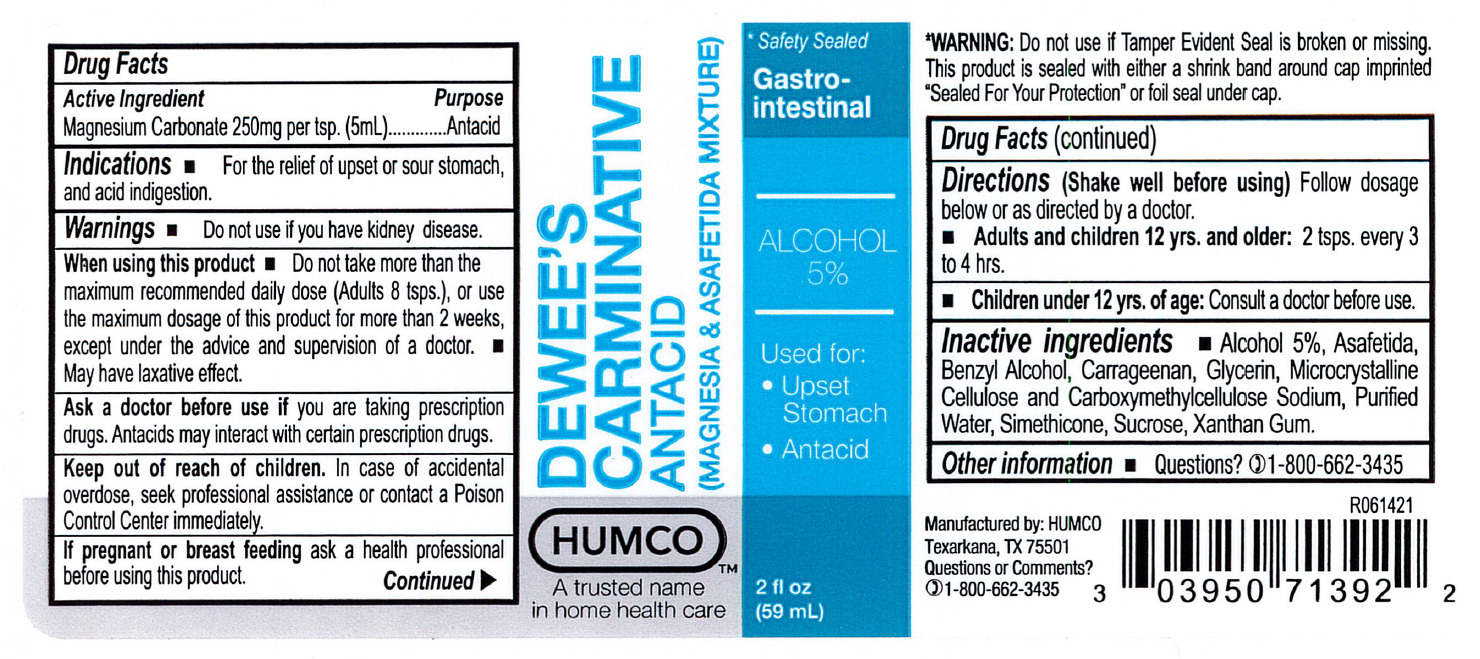
When sing this product
Do not take more than the maximum recommended daily dose (Adults 8 tsps. - children 4 tsps.), or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a doctor.
May have laxative effect.
Ask a doctor before use
If you are taking prescription drugs. Antacids may interact with certain prescription drugs.
Keep out of reach of children.
In the case of accidental overdose, seek professional assistance or contact a Poison Control center immediately.
Directions
(Shake well before using)
Follow dosage below or as directed by a doctor.
Adults and children 12 yrs. and older: 2 tsps. every 3 to 4 hrs.
Children under 12 yrs. of age: consult a doctor before use.

 New
New
