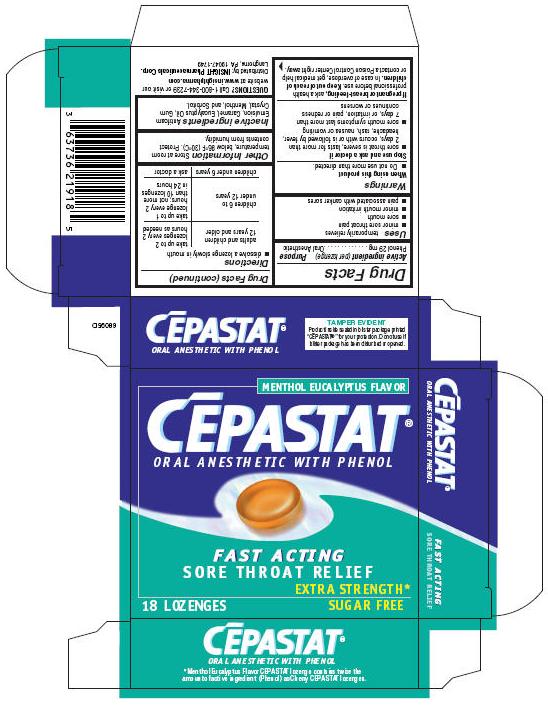
CEPASTAT EXTRA STRENGTH- phenol lozenge
Insight Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (per lozenge)
Phenol 29 mg
Uses
temporarily relieves
- minor sore throat pain
- sore mouth
- minor mouth irritation
- pain associated with canker sores
Warnings
When using this product
- Do not use more than directed.
Stop use and ask a doctor if
- sore throat is severe, lasts for more than 2 days, occurs with or is followed by fever, headache, rash, nausea or vomiting
- sore mouth symptoms last more than 7 days, or irritation, pain or redness continues or worsens
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- dissolve a lozenge slowly in mouth
| adults and children 12 years and older | take up to 2 lozenges every 2 hours as needed |
| children 6 to under 12 years | take up to 1 lozenge every 2 hours; not more than 10 lozenges in 24 hours |
| children under 6 years | ask a doctor |
Other Information
Store at room temperature, below 86°F (30°C). Protect contents from humidity.
Inactive ingredients
Antifoam Emulsion, Caramel, Eucalyptus Oil, Gum Crystal, Menthol, and Sorbitol.
QUESTIONS?
Call 1-800-344-7239 or visit our website at www.insightpharma.com
Distributed by: INSIGHT Pharmaceuticals Corp.
Langhorne, PA 19047-1749
PRINCIPAL DISPLAY PANEL - 18 Lozenges carton
MENTHOL EUCALYPTUS FLAVOR
CĒPASTAT®
ORAL ANESTHETIC WITH PHENOL
FAST ACTING
SORE THROAT RELIEF
EXTRA STRENGTH*
18 LOZENGES
SUGAR FREE