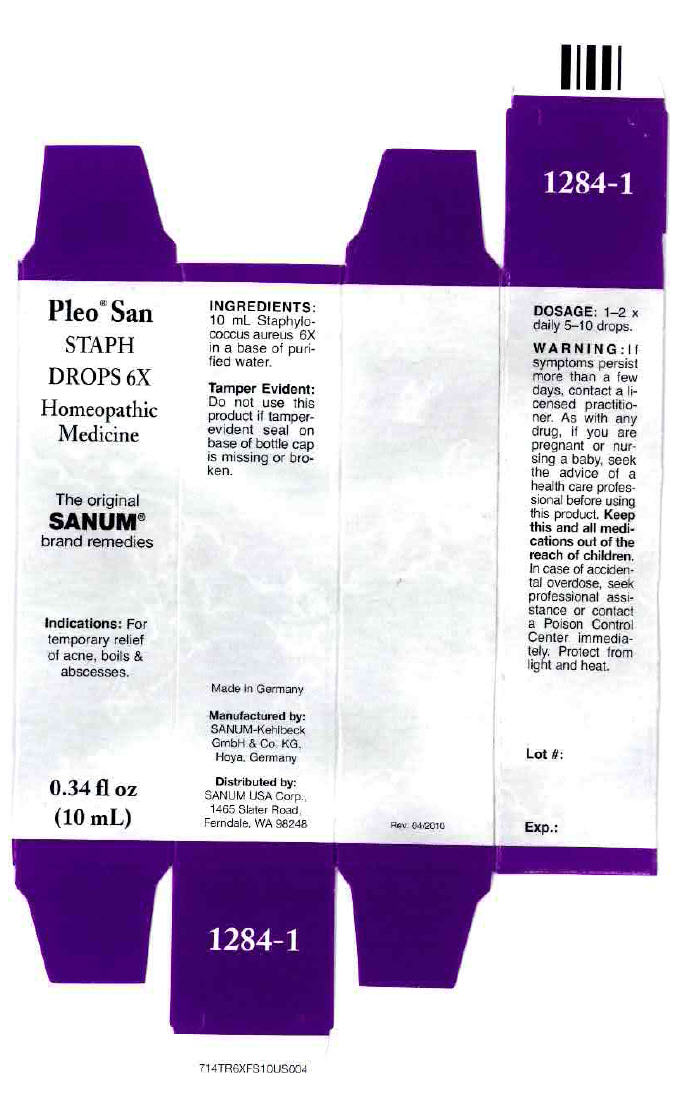
PLEO SAN STAPH- staphylococcus aureus solution/ drops
Sanum Kehlbeck GmbH & Co. KG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Homeopathic Medicine
0.34 fl oz
(10 mL)
Indications
For temporary relief of acne, boils & abscesses.
INGREDIENTS
10 mL Staphylococcus aureus 6X in a base of purified water.
Tamper Evident
Do not use this product if tamper-evident seal on base of bottle cap is missing or broken.
DOSAGE
1–2 × daily 5–10 drops.
WARNING
If symptoms persist more than a few days, contact a licensed practitioner. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health care professional before using this product.
Keep this and all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Protect from light and heat.
Made in Germany
Manufactured by:
SANUM-Kehlbeck
GmbH & Co. KG,
Hoya, Germany
Distributed by:
SANUM USA Corp.,
1465 Slater Road,
Ferndale, WA 98248
Rev. 04/2010
PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
Pleo® San
STAPH
DROPS 6X
Homeopathic
Medicine
The original
SANUM®
brand remedies
Indications: For
temporary relief
of acne, boils &
abscesses.
0.34 fl oz
(10 mL)
Sanum Kehlbeck GmbH & Co. KG