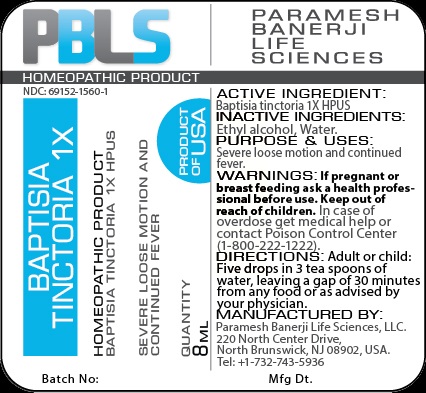
BAPTISIA TINCTORIA 1X- baptisia tinctoria liquid
Paramesh Banerji Life Sciences LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active Ingredient
Baptisia tinctoria 1X HPUS
Inactive Ingredients
Ethyl alcohol, Water
Purpose
Severe loose motion and continued fever
Uses
Severe loose motion and continued fever
Warnings
If pregnant or breast feeding ask a health professional before use.
Keep out of reach of children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Direction
Adult or child: Five drops in 3 tea spoons of water, leaving a gap of 30 minutes from any food or as advised by your physician.
Manufactured by
Paramesh Banerji Life Sciences, LLC
220 North Center Drive
North Brunswick, NJ 08902, USA
Tel: +1-732-743-5936
Principal Display Panel
Homeopathic Product
NDC: 69152-1560-1
Baptisia tinctoria 1X
Homeopathic Product
Baptisia tinctoria 1X HPUS
Severe loose motion and continued fever
Quantity
96 Pills
Product of USA
