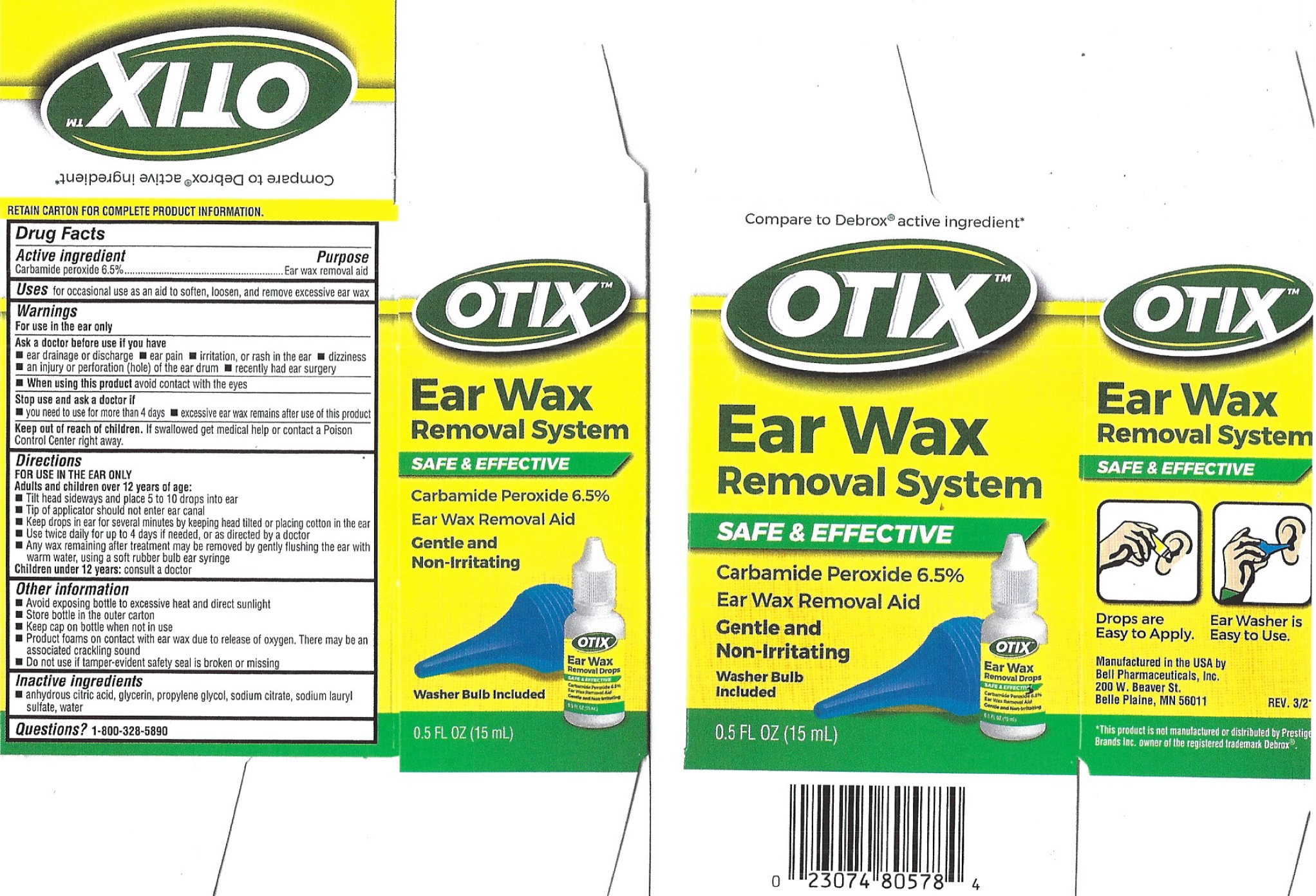
Warnings
For use in the ear only
Ask a doctor before use if you have
- ear drainage or discharge
- ear pain
- irritation, or rash in the ear
- dizziness
- an injury or perforation (hole) of the ear drum
- recently had ear surgery
Directions
FOR USE IN THE EAR ONLY
Adults and children over 12 years of age:
- Tilt head sideways and place 5 to 10 drops into ear
- Tip of applicator should not enter ear canal
- Keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear
- Use twice daily for up to 4 days if needed, or as directed by a doctor
- Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe Children Under 12 years: consult a doctor
Other information
- Avoid exposing bottle to excessive heat and direct sunlight
- Store bottle in the outer carton
- Keep cap in bottle when not in use
- Product foams on contact with earwax due to release of oxygen. There may be an assoiciated crackling sound.
- Do not use if tamper-evident safety seal is broken or missing