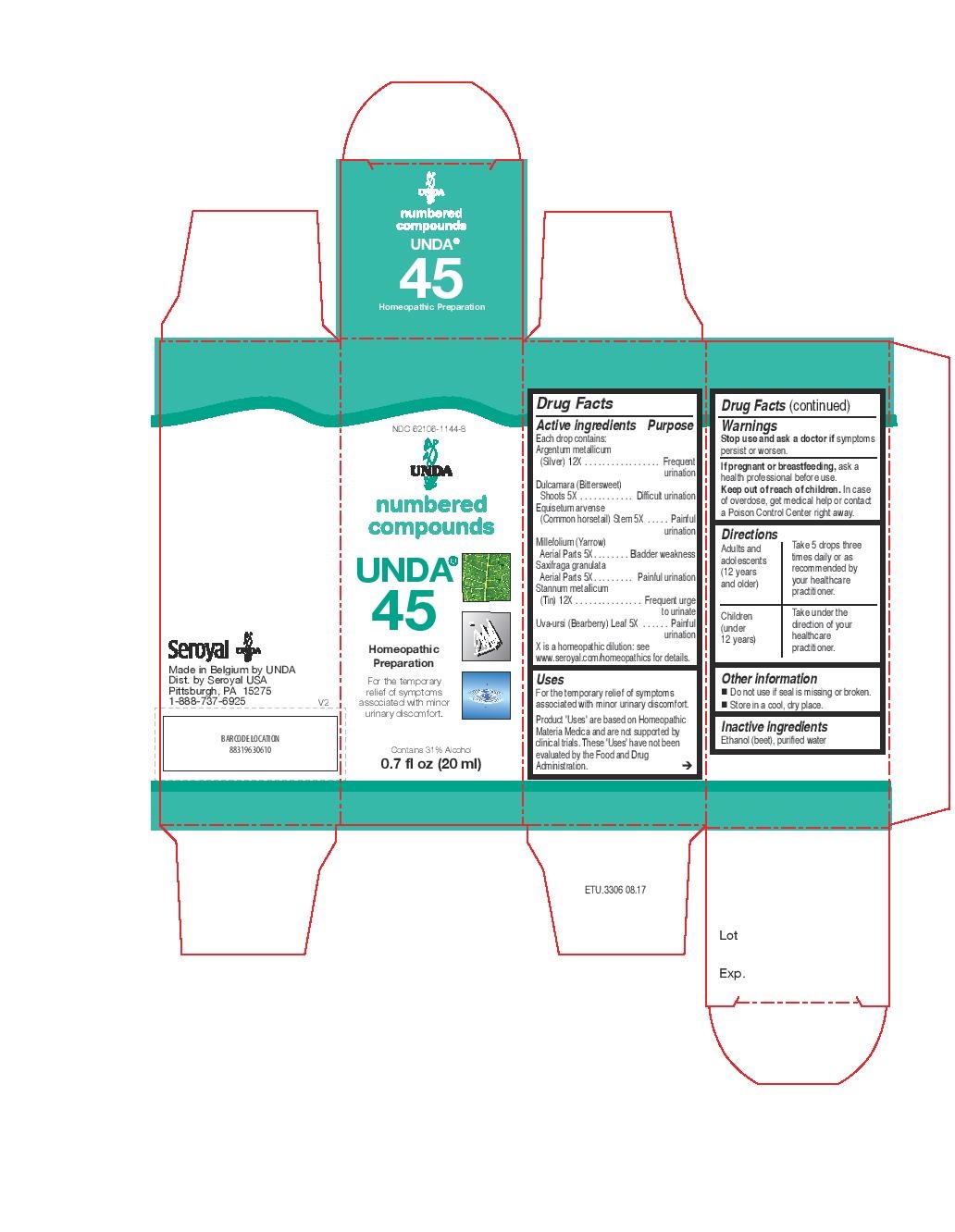
Active ingredients
Each drop contains:
Aluminium metallicum (Aluminum) 12X
Boldo Leaf 4X
Chamomilla (German chamomile) Whole Flowering Plant 4X
Condurango (Eagle vine) Bark 4X
Equisetum arvense (Common horsetail) Stem 4X
Stannum metallicum (Tin) 12X
Viola tricolor (Pansy) Aerial Parts 4X
Warnings
Stop use and ask a doctor if symptoms
persist or worsen.
If pregnant or breastfeeding, ask a
health professional before use.
Keep out of reach of children. In case
of overdose, get medical help or contact
a Poison Control Center right away.
Uses
For the temporary relief of symptoms associated with abdominal cramping, bloating and gas following meals.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms associated with abdominal cramping, bloating and gas following meals.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Active ingredients
Each drop contains:
Argentum metallicum (Silver) 12X
Cytisus scoparius (Scotch broom) Aerial Parts 4X
Equisetum arvense (Common horsetail) Stem 4X
Hieracium pilosella (Mouse-ear hawkweed) Whole Plant 4X
Juniperus communis (Common juniper) Berry 4X
Uva-ursi (Bearberry) Leaf 4X
Uses
For the temporary relief of symptoms
associated with difficult urination
and occasional incontinence.
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a
Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms
associated with difficult urination
and occasional incontinence.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Active ingredients
Each drop contains:
Saxifraga granulata Aerial Parts. 4X
Origanum vulgare Aerial Parts. 4X
Lactuca virosa (Poisonous lettuce) Whole Plant . 4X
Veronica officinalis (Gypsyweed) Aerial Parts . 4X
Ajuga reptansWhole Plant. 4X
Drosera (Sundew)Whole Plant . 4X
Senega officinalis Root . 4X
Aurum metallicum (Gold) . 12X
Argentum metallicum (Silver) . 12X
Indications
To help with calcium absorption and for symptoms associated
with minor respiratory problems.
Warnings
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a
Poison Control Center right away.
If symptoms persist or worsen, consult your healthcare practitioner.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Indications
To help with calcium absorption and for symptoms associated
with minor respiratory problems.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Active ingredients
Each drop contains:
Argentum metallicum (Silver) 12X
Dulcamara (Bittersweet) Shoots 5X
Equisetum arvense (Common horsetail) Stem 5X
Millefolium (Yarrow) Aerial Parts 5X
Saxifraga granulata Aerial Parts 5X
Stannum metallicum (Tin) 12X
Uva-ursi (Bearberry) Leaf 5X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact
a Poison Control Center right away.
Other information
Do not use if seal is missing or broken.
Store in a cool, dry place.
Contents may not fill package in order
to accommodate required labeling.
Please rely on stated quantity.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of symptoms associated with minor urinary discomfort.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
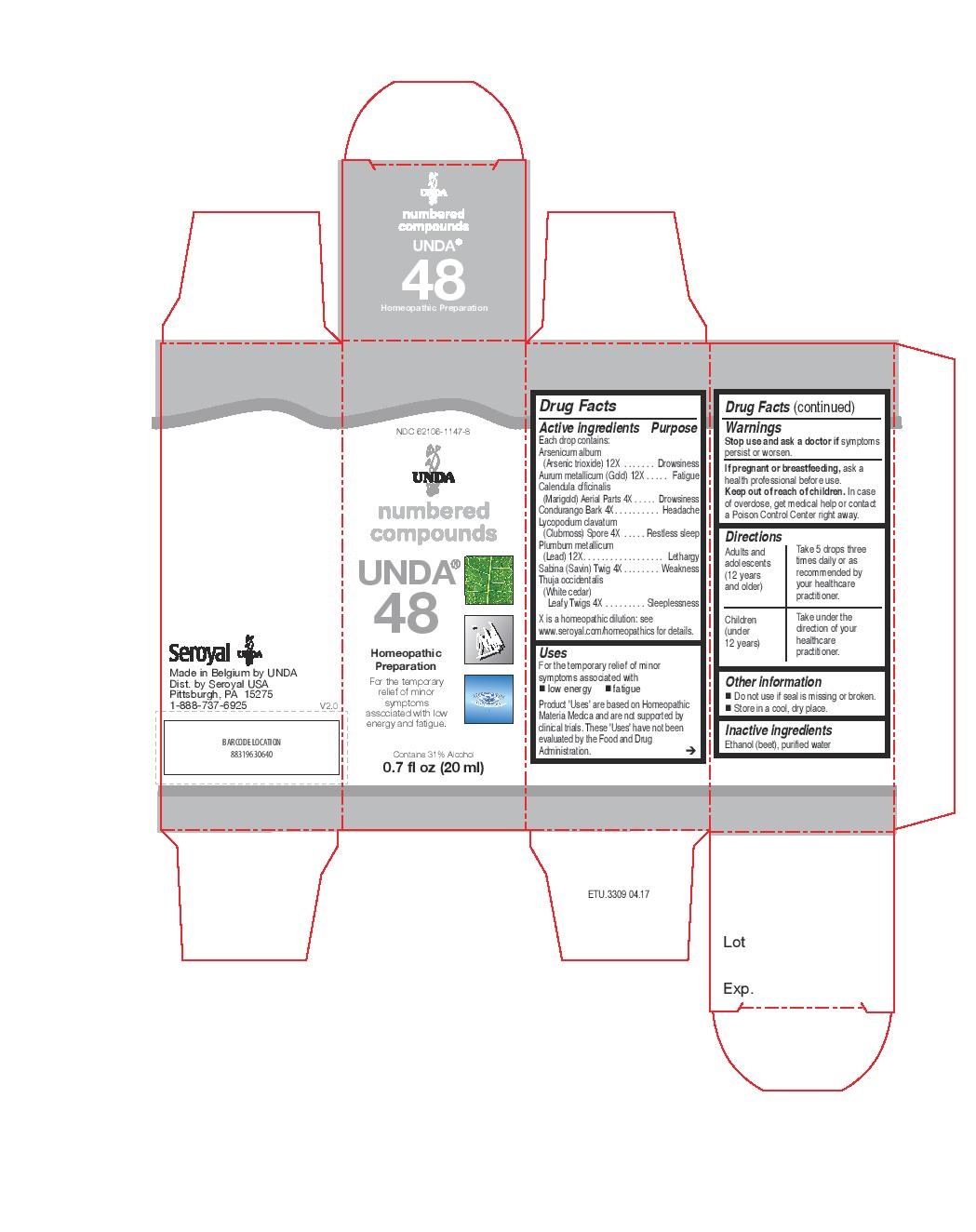
Active ingredients
Each drop contains:
Arsenicum album (Arsenic trioxide) 12X
Aurum metallicum (Gold) 12X
Calendula officinalis (Marigold) Aerial Parts 4X
Condurango Bark 4X
Lycopodium clavatum (Clubmoss) Spore 4X
Plumbum metallicum (Lead) 12X
Sabina (Savin) Twig 4X
Thuja occidentalis (White cedar) Leafy Twigs 4X
Warnings
Stop use and ask a doctor if symptoms persist or worsen.
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
Uses
For the temporary relief of minor symptoms associated with low energy and fatigue.
Directions
Adults and adolescents (12 years and older)
Take 5 drops three times daily or as recommended by your healthcare practitioner
Children (under 12 years)
Take under the direction of your healthcare practitioner.
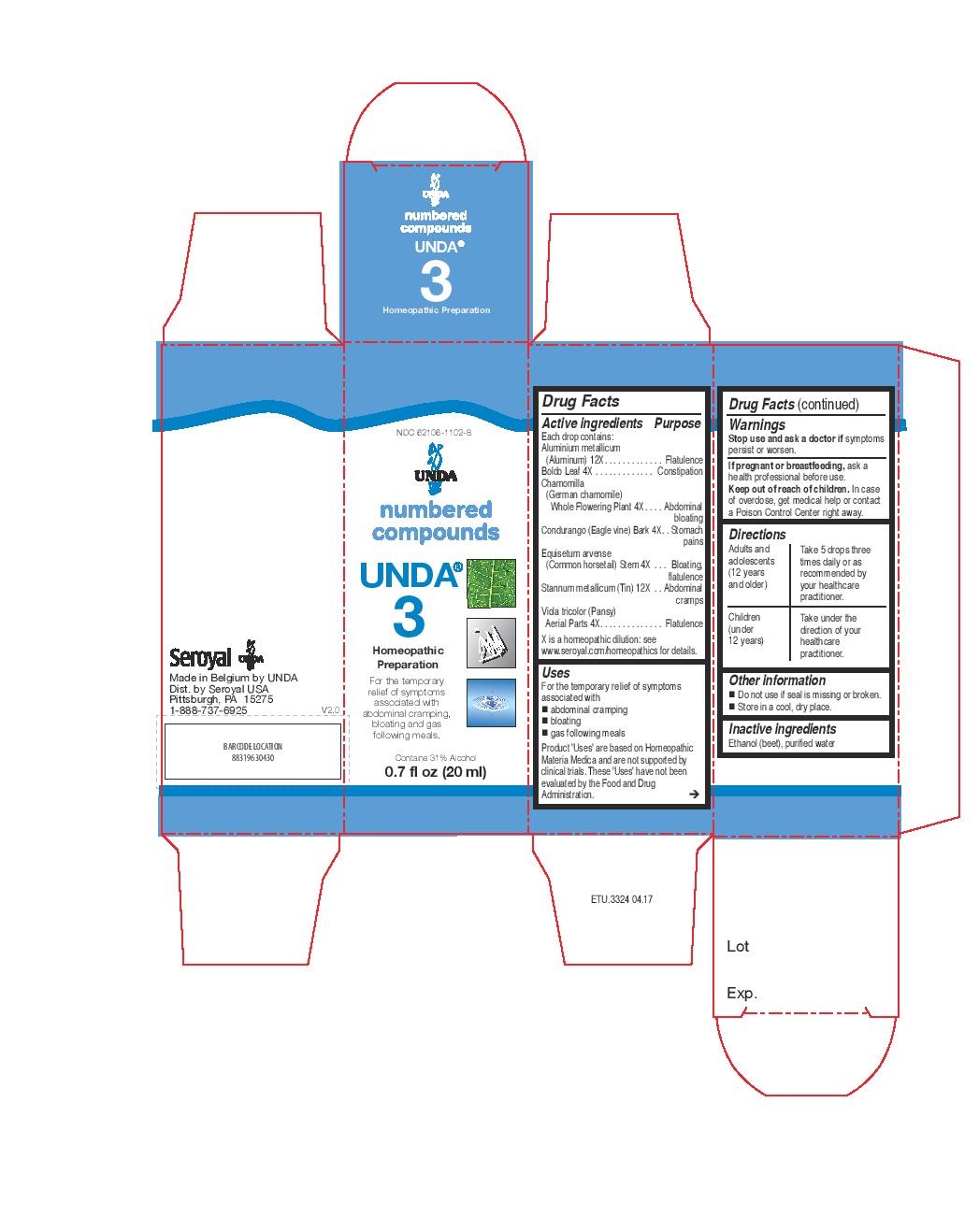
NDC 62106-1102-8
UNDA
numbered compounds
UNDA 3
Homeopathic Preparation
For the temporary relief of symptoms associated with abdominal cramping, bloating and gas following meals.
Contains 31% Alcohol
0.7 fl oz (20 ml)
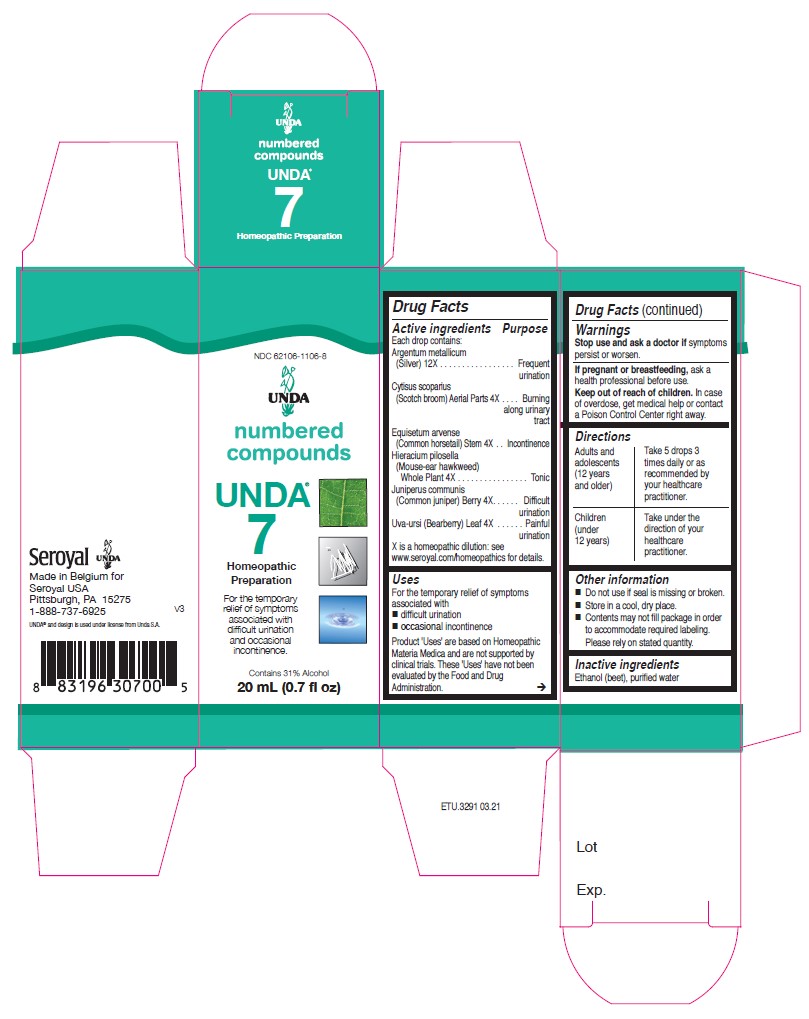
NDC 62106-1106-8
UNDA
numbered compounds
UNDA 7
Homeopathic Preparation
For the temporary
relief of symptoms
associated with
difficult urination
and occasional
incontinence.
Contains 31% Alcohol
0.7 fl oz (20 ml)
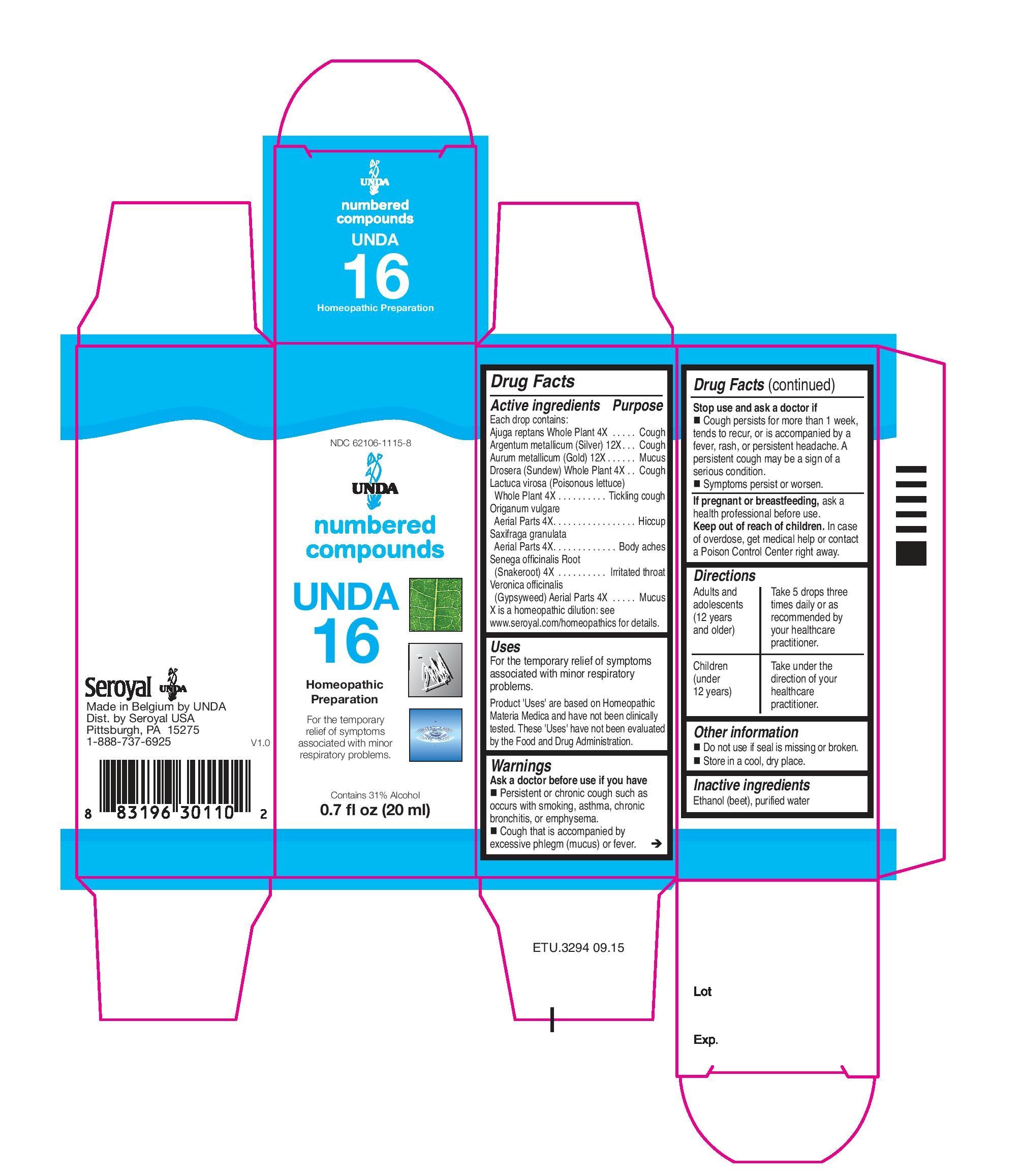
UNDA
numbered compounds
NDC 62106-1115-8
Unda 16
Homeopathic Preparation
For the temporary relief of symptoms associated with minor respiratory problems.
Contains 31% Alcohol
0.7 fl oz (20 ml)