FULL PRESCRIBING INFORMATION
1. INDICATIONS AND USAGE
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-month administration (leuprolide acetate) is indicated for treatment of advanced prostate cancer.
2. DOSAGE AND ADMINISTRATION
2.1 LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-Month Administration
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION must be administered under the supervision of a physician.
In patients treated with GnRH analogues for prostate cancer, treatment is usually continued upon development of metastatic castration-resistant prostate cancer.
| Dosage | 22.5 mg for 3-Month Administration |
| Recommended Dose | 1 injection every 12 weeks |
The recommended dose of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-month administration is one injection every 12 weeks. Do not use concurrently a fractional dose, or a combination of doses of this or any depot formulation due to different release characteristics.
Incorporated in a depot formulation, the lyophilized microspheres must be reconstituted and administered every 12 weeks as a single intramuscular injection.
2.2 Reconstitution Instructions for LEUPROLIDE ACETATE FOR DEPOT SUSPENSION
- Reconstitute and administer the lyophilized microspheres as a single intramuscular injection.
- The suspension should be administered immediately after reconstitution.
- As with other drugs administered by intramuscular injection, the injection site should be alternated periodically.
- Visually inspect LEUPROLIDE ACETATE FOR DEPOT SUSPENSION powder (white to off-white powder). DO NOT USE the vial if clumping or caking is evident. A thin layer of powder on the wall of the vial is considered normal prior to mixing with the diluent. The diluent contained in the prefilled syringe should appear clear and colorless.
- Use ONLY the provided diluent for reconstitution of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION. DO NOT use other diluents.
- The reconstituted product is a suspension of milky, white color appearance.
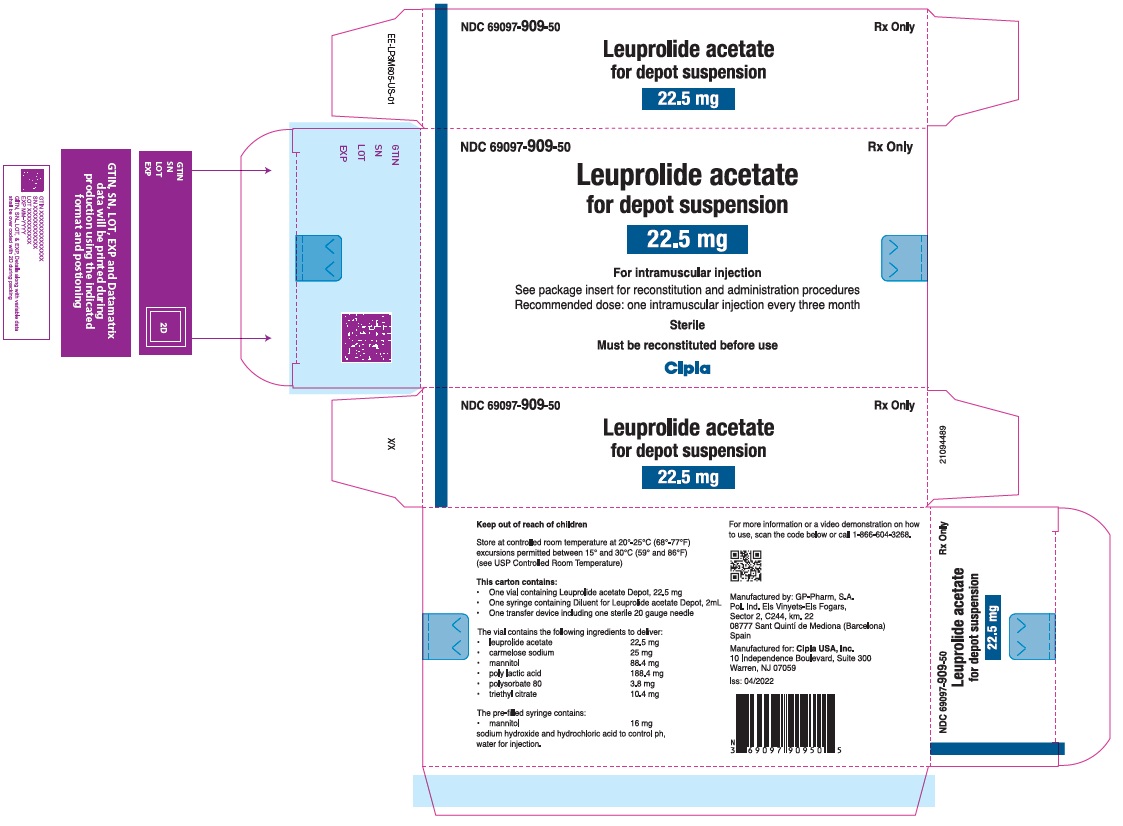
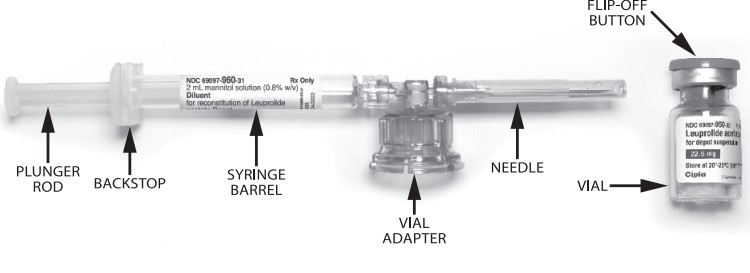
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION is packaged in a commercial kit. Each kit contains:
- One vial containing 22.5 mg of leuprolide acetate as lyophilized microspheres.
- One prefilled syringe containing 2 mL of mannitol for injection.
- One MIXJECT transfer device including one needle.
 |
Please read the instructions completely before you begin.
MIXJECT Preparation
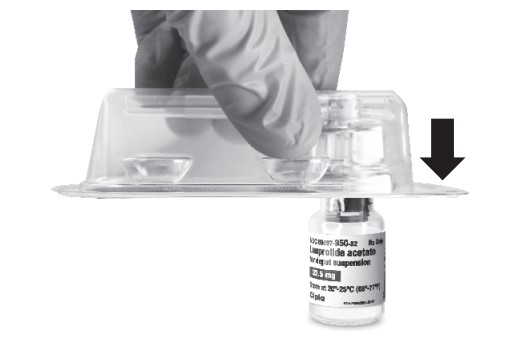
Wash your hands with soap and hot water and put on gloves1 immediately prior to preparing the injection. Place the tray on a clean, flat surface that is covered with a sterile pad or cloth. Remove the MIXJECT device, the backstop, the prefilled syringe containing the solvent for reconstitution and the LEUPROLIDE ACETATE FOR DEPOT SUSPENSION vial.
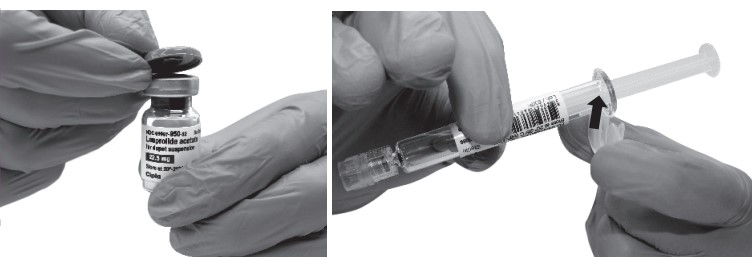
Remove the Flip-Off button from the top of the vial, revealing the rubber stopper. Place the vial in a standing upright position on the prepared surface. Disinfect the rubber stopper with the alcohol wipe. Discard the alcohol wipe and allow the stopper to dry. Insert the backstop to the flange of the syringe until you feel it snap in place. Proceed to MIXJECT Activation.
 |
MIXJECT Activation
- Peel the cover away from the blister pack containing the vial adapter (MIXJECT). Do not remove the vial adapter from the blister pack. Place the blister pack containing the vial adapter firmly on the vial top, piercing the vial. Push down gently until you feel it snap in place.
- Remove the cap from the syringe barrel and then, remove the blister pack from the vial adapter. Connect the syringe to the vial adapter by screwing it clockwise into the opening on the side of the vial adapter. Be sure to gently twist the syringe until it stops turning to ensure a tight connection.
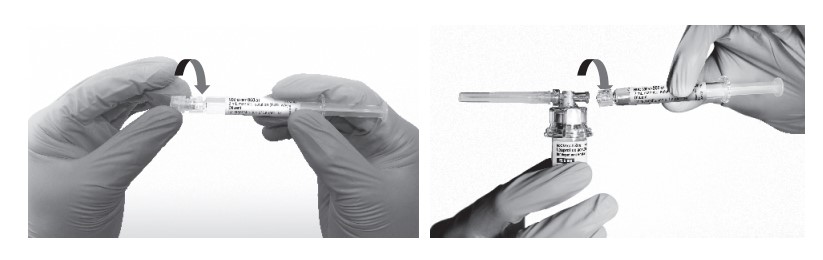
- While holding the vial, place your thumb on the plunger rod and push the plunger rod in all the way to transfer the diluent from the pre-filled syringe into the vial. Do not release the plunger rod.
-
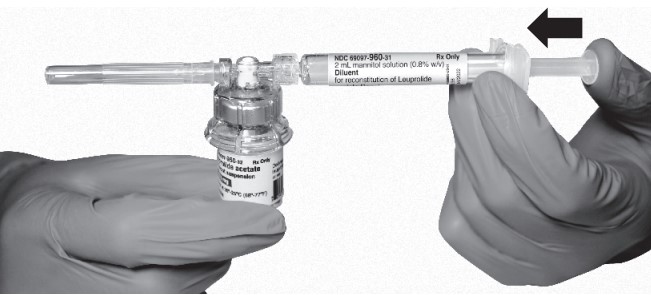
Keeping the plunger rod depressed, gently swirl the vial for approximately one minute until a uniform milky-white suspension is obtained. This will ensure complete mixing of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION and the sterile mannitol solution diluent. The suspension will now have a milky appearance. In order to avoid separation of the suspension, proceed to the next steps without delay.
- Invert the MIXJECT system so that the vial is at the top. Grasp the MIXJECT system firmly by the syringe and pull back the plunger rod slowly to draw the reconstituted LEUPROLIDE ACETATE FOR DEPOT SUSPENSION into the syringe.
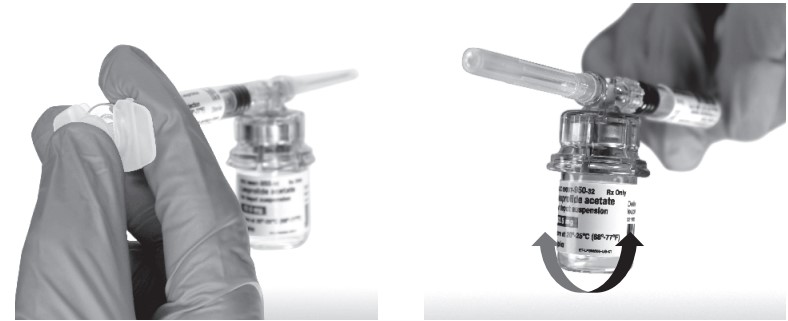
Return the vial to its upright position, and disconnect the vial adapter from the MIXJECT syringe assembly by grabbing firmly the syringe and turning the plastic cap of the vial adapter clockwise. Grasp only the plastic cap when removing.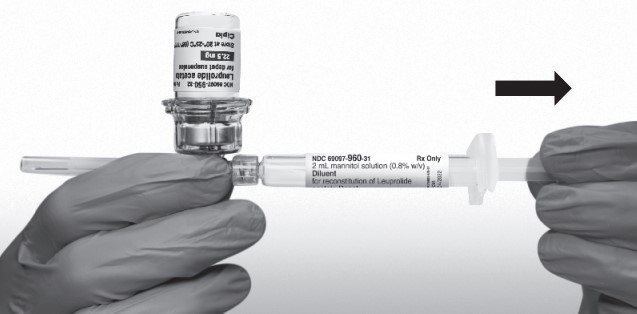
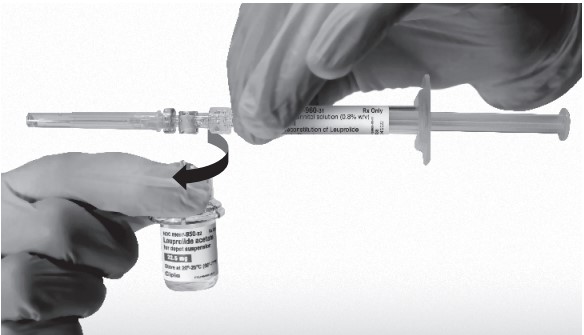
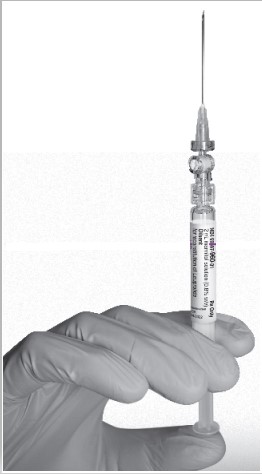
- Keep the syringe UPRIGHT. With the opposite hand pull the needle cap upward. Advance the plunger to expel the air from the syringe. The syringe containing LEUPROLIDE ACETATE FOR DEPOT SUSPENSION is now ready for administration. The suspension should be administered immediately after reconstitution.
- After cleaning the injection site with an alcohol swab, administer the intramuscular injection by inserting the needle at a 90 degree angle into the gluteal area, anterior thigh, or deltoid; injection sites should be alternated (see Figure).
NOTE: If a blood vessel is accidentally penetrated, aspirated blood will be visible just below the luer lock. If blood is present, remove the needle immediately. Do not inject the medication.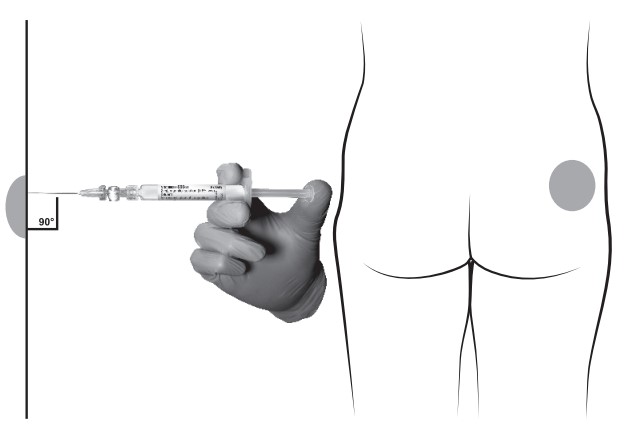
- Inject the entire contents of the syringe intramuscularly.
- Withdraw the needle. Once the syringe has been withdrawn, immediately discard the needle into a suitable sharps container. Dispose the syringe according to local regulations/procedures.
3. DOSAGE FORMS AND STRENGTHS
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION
For Injection: 22.5 mg of leuprolide acetate for 3-month administration as lyophilized microspheres in a single dose vial as a kit with a prefilled syringe containing 2mL 0.8% mannitol solution and a MIXJECT transfer device for a single dose injection.
4. CONTRAINDICATIONS
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION is contraindicated in:
-
Hypersensitivity
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION is contraindicated in individuals with known hypersensitivity to GnRH agonists or any of the excipients in LEUPROLIDE ACETATE FOR DEPOT SUSPENSION. Reports of anaphylactic reactions to GnRH agonists have been reported in the medical literature.
5. WARNINGS AND PRECAUTIONS
5.1 Tumor Flare
Initially, LEUPROLIDE ACETATE FOR DEPOT SUSPENSION, like other GnRH agonists, causes increases in serum levels of testosterone to approximately 50% above baseline during the first weeks of treatment. Isolated cases of ureteral obstruction and spinal cord compression have been observed, which may contribute to paralysis with or without fatal complications. Transient worsening of symptoms may develop. A small number of patients may experience a temporary increase in bone pain, which can be managed symptomatically.
Patients with metastatic vertebral lesions and/or with urinary tract obstruction should be closely observed during the first few weeks of therapy.
5.2 Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
5.3 Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
5.4 Effect on QT/QTc Interval
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
5.5 Convulsions
Postmarketing reports of convulsions have been observed in patients on leuprolide acetate therapy. These included patients with a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs. Convulsions have also been reported in patients in the absence of any of the conditions mentioned above. Patients receiving a GnRH agonist who experience convulsion should be managed according to current clinical practice.
5.6 Laboratory Tests
Monitor serum levels of testosterone following injection of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-month administration. In the majority of patients, testosterone levels increased above baseline during the first week, and then declined thereafter to castrate levels (< 50 ng/dL) within four weeks. [see Clinical Studies (14) and Adverse Reactions (6)].
5.7 Embryo-Fetal Toxicity
Based on findings in animal studies, LEUPROLIDE ACETATE FOR DEPOT SUSPENSION may cause fetal harm when administered to a pregnant woman. In animal developmental and reproductive toxicology studies, administration of the monthly formulation of leuprolide acetate on day 6 of pregnancy (sustained exposure was expected throughout the period of organogenesis) caused adverse embryo-fetal toxicity in animals at doses less than the human dose, based on body surface area, using an estimated daily dose. Advise pregnant patients and females of reproductive potential of the potential risk to the fetus [see Use in Specific Populations (8.1)].
6. ADVERSE REACTIONS
The following is discussed in more detail in other sections of the labeling:
- Tumor Flare [see Warnings and Precautions (5.1)]
- Hyperglycemia and Diabetes [see Warnings and Precautions (5.2)]
- Cardiovascular Disease [see Warnings and Precautions (5.3)]
- Effect on QT/QTc Interval [see Warnings and Precautions (5.4)]
- Convulsions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-Month Administration
In a clinical trial of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-month administration, patients were treated for 24 weeks with 157/163 receiving two injections. The table includes adverse reactions were reported in 5% or more of the patients during the treatment period as well as the incidence of these adverse reaction that were considered, by the treating physician, to be at least possibly related to LEUPROLIDE ACETATE FOR DEPOT SUSPENSION. Grade 3-4 adverse reactions reported as treatment-emergent in 13% of patients and treatment-related 4% of patients.
|
CCTCAE v.3 1 Includes cold sweat, flushing, hot flush, hyperhidrosis, and night sweats 2Includes influenza, influenza-like illness, nasal congestion, nasopharyngitis, rhinorrhea, upper respiratory tract infection and congestion |
||
| Table 2. Adverse Reactions Reported in ≥ 5% of Patients | ||
| LEUPROLIDE ACETATE FOR DEPOT SUSPENSION
22.5 mg for 3-Month Administration
|
||
| Grade 1-4 | ||
| Treatment-Emergent | Treatment-Related | |
| Hot Flush/Flushing1 | 128 (79) | 127 (78) |
| Upper Respiratory Infection2 | 28 (17) | 0 |
| Fatigue/Asthenia | 24 (15) | 22 (13) |
| Diarrhea | 21 (13) | 2 (1) |
| Pollakiuria | 20 (12) | 3 (2) |
| Arthralgia/Arthritis | 18 (11) | 2 (1) |
| Injection Site Pain/Discomfort | 18 (11) | 15 (9) |
| Constipation | 15 (9) | 1 (0.6) |
| Extremity Pain | 14 (9) | 0 |
| Nausea | 14 (9) | 4 (2) |
| Nocturia | 14 (9) | 3 (2) |
| Abdominal Pain/Discomfort | 13 (8) | 1 (0.6) |
| Urinary Tract Pain | 13 (8) | 2 (1) |
| Dizziness | 12 (7) | 2 (1) |
| Headache/Sinus Headache | 12 (7) | 1 (0.6) |
| Urinary Tract Infection | 12 (7) | 0 |
| Bone Pain | 11 (7) | 4 (2) |
| Back Pain | 10 (6) | 1 (0.6) |
| Hypertension/Blood Pressure Increased | 10 (6) | 0 |
| Pruritus/Generalized Pruritus | 9 (6) | 3 (2) |
In the same study, erectile dysfunction and testicular atrophy were reported in patients on LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg.
Laboratory abnormalities
During the treatment period, at least a one grade change in laboratory values was seen (>10%) in the following: anemia, increased triglyceride, hyperglycemia, increased cholesterol, increased creatine kinase, leukopenia, increased AST, increased creatinine, and increased ALT.
6.2 Post-marketing Experience
The following adverse reactions have been identified during post approval use of gonadotropin-releasing hormone agonists. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Pituitary apoplexy: During postmarketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
Changes in Bone Density: Decreased bone density has been reported in the medical literature in men who have had orchiectomy or who have been treated with a GnRH agonist analog. In a clinical trial, 25 men with prostate cancer, 12 of whom had been treated previously with leuprolide acetate for at least six months, underwent bone density studies as a result of pain. The leuprolide-treated group had lower bone density scores than the nontreated control group. It can be anticipated that long periods of medical castration in men will have effects on bone density.
Immune Disorders: Anaphylaxis
Psychiatric Disorders: Depression
Respiratory Disorders: Pneumonitis, Interstitial Lung Disease
7. DRUG INTERACTIONS
No pharmacokinetic-based drug-drug interaction studies have been conducted with LEUPROLIDE ACETATE FOR DEPOT SUSPENSION.
7.1 Drug/Laboratory Test Interactions
Administration of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION in therapeutic doses results in suppression of the pituitary-gonadal system. Normal function is usually restored within three months after treatment is discontinued. Due to the suppression of the pituitary-gonadal system by LEUPROLIDE ACETATE FOR DEPOT SUSPENSION, diagnostic tests of pituitary gonadotropic and gonadal functions conducted during treatment and up to three months after discontinuation of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION may be affected.
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Risk Summary
Based on findings in animal studies and mechanism of action, LEUPROLIDE ACETATE FOR DEPOT SUSPENSION may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal developmental and reproductive toxicology studies, administration of a monthly formulation of leuprolide acetate on day 6 of pregnancy (sustained exposure was expected throughout the period of organogenesis) caused adverse embryo-fetal toxicity in animals at doses less than the human dose based on body surface area using an estimated daily dose (see data). Advise pregnant patients and females of reproductive potential of the potential risk to the fetus.
Animal Data
Major fetal malformations were observed in developmental and reproductive toxicology studies in rabbits after a single administration of the monthly formulation of leuprolide acetate on day 6 of pregnancy at doses of 0.00024, 0.0024, and 0.024 mg/kg (approximately 1/1600 to 1/16 the human dose based on body surface area using an estimated daily dose in animals and humans). Since a depot formulation was utilized in the study, a sustained exposure to leuprolide was expected throughout the period of organogenesis and to the end of gestation. Similar studies in rats did not demonstrate an increase in fetal malformations, however, there was increased fetal mortality and decreased fetal weights with the two higher doses of the monthly formulation of leuprolide acetate in rabbits and with the highest dose (0.024 mg/kg) in rats.
8.2. Lactation
The safety and efficacy of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION have not been established in females. There is no information regarding the presence of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION in human milk, the effects on the breastfed child, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in a breastfed child from LEUPROLIDE ACETATE FOR DEPOT SUSPENSION, a decision should be made to discontinue breastfeeding or discontinue the drug, taking into account the importance of the drug to the mother.
8.3. Females and Males of Reproductive Potential
Infertility
Males
Based on findings in animals and mechanism of action, LEUPROLIDE ACETATE FOR DEPOT SUSPENSION may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
10. OVERDOSAGE
There is no experience of over dosage in clinical trials. In rats, a single subcutaneous dose of 100 mg/kg (approximately 4,000 times the estimated daily human dose based on body surface area), resulted in dyspnea, decreased activity, and excessive scratching.
In early clinical trials with daily subcutaneous leuprolide acetate, doses as high as 20 mg/day for up two years caused no adverse effects differing from those observed with the 1 mg/day dose.
11. DESCRIPTION
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin-releasing hormone (GnRH). The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
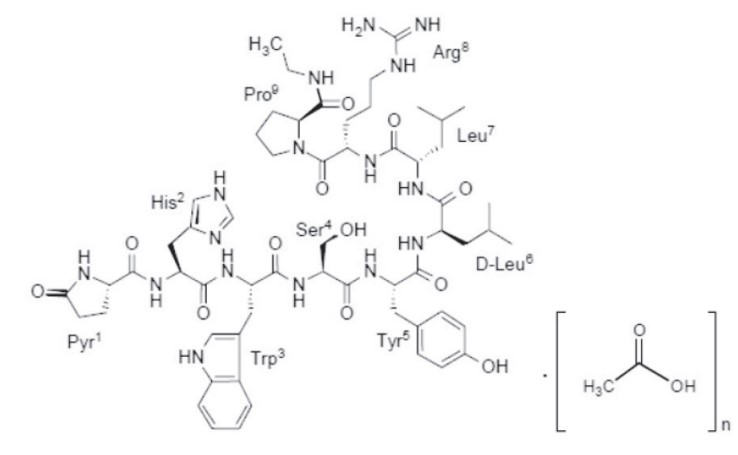
Where: n=1 or 2
Leuprolide acetate has a molecular weight of 1209.41 as free base”. Leuprolide is freely soluble in water.
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-month administration is available in a vial containing white to off white sterile lyophilized microspheres together with the corresponding sterile reconstitution diluent in a pre-filled syringe. When LEUPROLIDE ACETATE FOR DEPOT SUSPENSION and the diluent are mixed together they become a suspension intended as an intramuscular injection to be given ONCE EVERY 12 WEEKS as a single dose.
Each vial of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-month administration delivers leuprolide acetate (22.5 mg), polylactic acid (188.4 mg), triethylcitrate (10.4 mg), polysorbate 80 (3.8 mg), mannitol (88.4 mg) and carmellose sodium (25 mg). The prefilled syringe containing the clear reconstitution diluent (2 mL) contains mannitol (16 mg), water for injections, and sodium hydroxide and hydrochloric acid to control pH.
Leuprolide acetate for depot suspension is an extended release sterile, single dose injection in suspension form for intramuscular administration.
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Leuprolide acetate, a GnRH agonist, acts as an inhibitor of gonadotropin secretion. Animal studies indicate that following an initial stimulation, continuous administration of leuprolide acetate results in suppression of ovarian and testicular steroidogenesis. This effect was reversible upon discontinuation of drug therapy.
Administration of leuprolide acetate has resulted in inhibition of the growth of certain hormone dependent tumors (prostate tumors in Noble and Dunning male rats and DMBA-induced mammary tumors in female rats) as well as atrophy of the reproductive organs.
12.2 Pharmacodynamics
In humans, administration of leuprolide acetate results in an initial increase in circulating levels of luteinizing hormone (LH) and follicle stimulating hormone (FSH), leading to a transient increase in levels of the gonadal steroids (testosterone and dihydrotestosterone in males, and estrone and estradiol in premenopausal females). However, continuous administration of leuprolide acetate results in decreased levels of LH and FSH. In males, testosterone is reduced to below castrate threshold. In premenopausal females, estrogens are reduced to postmenopausal concentrations. These decreases occur within two to four weeks after initiation of treatment. Long-term studies have shown that continuation of therapy with leuprolide acetate maintains testosterone below the castrate level for more than five years.
Leuprolide acetate is not active when given orally.
12.3 Pharmacokinetics
Absorption
Following two sequential injections of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg administered with a 3-month interval, plasma leuprolide concentrations were similar among both cycles. After first administration, mean plasma leuprolide concentration of 46.8 ng/mL was observed at approximately 2 hours and the mean concentration then declined until next injection.
Distribution
The mean steady-state volume of distribution of leuprolide following intravenous bolus administration to healthy male volunteers was 27 L. In vitro binding to human plasma proteins ranged from 43% to 49%.
Elimination
The mean systemic clearance of leuprolide following intravenous bolus administration to healthy male volunteers was 7.6 L/h, and terminal elimination half-life was approximately 3 hours based on a two-compartment model.
Upon administration with different leuprolide acetate formulations, the major metabolite of leuprolide acetate is a pentapeptide (M- 1) metabolite.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted with leuprolide acetate in rats and mice. In rats, a dose-related increase of benign pituitary hyperplasia and benign pituitary adenomas was noted at 24 months when the drug was administered subcutaneously at high daily doses (0.6 to 4 mg/kg). There was a significant but not dose-related increase of pancreatic islet cell adenomas in females and of testicular interstitial cell adenomas in males (highest incidence in the low dose group). In mice, no leuprolide acetate-induced tumors or pituitary abnormalities were observed at a dose as high as 60 mg/kg for two years. Patients have been treated with leuprolide acetate for up to three years with doses as high as 10 mg/day and for two years with doses as high as 20 mg/day without demonstrable pituitary abnormalities. No carcinogenicity studies have been conducted with LEUPROLIDE ACETATE FOR DEPOT SUSPENSION.
Genotoxicity studies were conducted with leuprolide acetate using bacterial and mammalian systems. These studies provided no evidence of mutagenic effects or chromosomal aberrations.
Leuprolide may reduce male and female fertility. Administration of leuprolide acetate to male and female rats at dose of 0.024, 0.24, and 2.4 mg/kg as monthly depot formulation for up to 3 months (approximately as low as 1/30 of the human dose based on body surface area using an estimated daily dose in animals and humans) caused atrophy of the reproductive organs, and suppression of reproductive function. These changes were reversible upon cessation of treatment.
14 CLINICAL STUDIES
14.1 LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg for 3-Month Administration
The efficacy of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg was evaluated in an open-label, multicenter, non-controlled, multiple dose clinical trial which enrolled 162 evaluable patients with prostate cancer. Patients were administered LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg intramuscularly in 2 doses (157 received 2 injections) with a 3-month interval.
The median age was 71 years (range; 47-91), 62% White, and 30% Black or African-American.
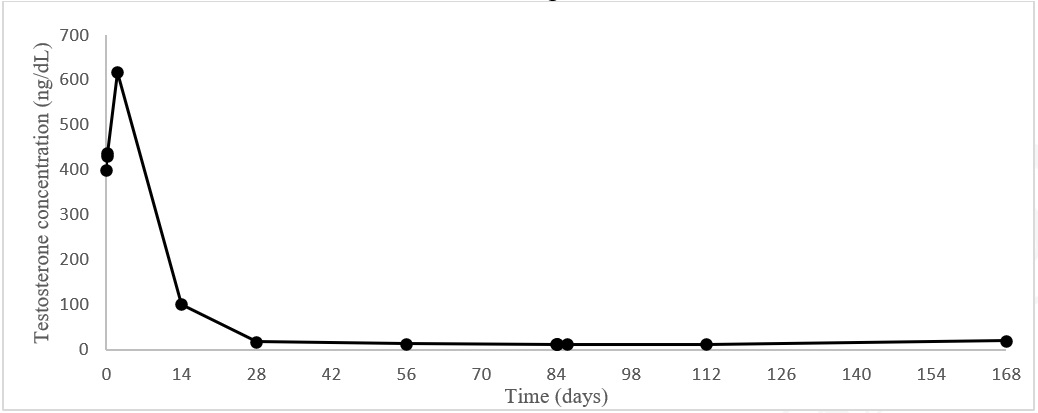
Castrate levels of serum testosterone (<50 ng/dL) were achieved and maintained from Day 28 to 168 in 94.3% (95% CI:89.4, 97.0) of patients. On Day 28, 160 of the 162 (98.8%) patients had castrate testosterone levels. One patient did not achieve a castrate level and one had a missing value. Testosterone escapes (any value > 50 ng/dL after castrate levels were achieved) occurred in four patients. In addition, three patients had a single unevaluable testosterone level after Day 28 that was considered to be non-castrate in this analysis.
Figure 1. Mean testosterone plasma levels during treatment with two three-month IM injections of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg
16 HOW SUPPLIED/STORAGE AND HANDLING
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION is supplied as a kit consisting of a LEUPROLIDE ACETATE FOR DEPOT SUSPENSION single-dose delivery system consisting of a vial with a Flip-Off seal containing sterile, white to off white lyophilized leuprolide acetate microspheres incorporated in a biodegradable polymer, a MIXJECT vial adapter containing the needle, and a pre-filled syringe containing clear sterile mannitol solution for injection, USP, 2 mL, pH 4.5 to 7.0.
LEUPROLIDE ACETATE FOR DEPOT SUSPENSION 22.5 mg – NDC 69097-909-50
Storage
Store at controlled room temperature at 20º-25ºC (68º-77ºF); excursions permitted between 15ºC and 30ºC (59ºC and 86ºF) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
Hypersensitivity
- Inform patients that if they have experienced hypersensitivity with other GnRH agonist drugs like LEUPROLIDE ACETATE FOR DEPOT SUSPENSION, LEUPROLIDE ACETATE FOR DEPOT SUSPENSION is contraindicated [see Contraindications (4)].
Tumor Flare
- Inform patients that LEUPROLIDE ACETATE FOR DEPOT SUSPENSION can cause tumor flare during the first weeks of treatment. Inform patients that the increase in testosterone can cause an increase in urinary symptoms or pain. Advise patients to contact their healthcare provider if uretral obstruction, spinal cord compression, paralysis, or new or worsened symptoms occur after beginning LEUPROLIDE ACETATE FOR DEPOT SUSPENSION treatment [see Warnings and Precautions (5.1)].
Hyperglycemia and Diabetes
- Advise patients that there is an increased risk of hyperglycemia and diabetes with LEUPROLIDE ACETATE FOR DEPOT SUSPENSION therapy. Inform patients that periodic monitoring for hyperglycemia and diabetes is required when being treated with LEUPROLIDE ACETATE FOR DEPOT SUSPENSION [see Warnings and Precautions (5.2)].
Cardiovascular Disease
- Inform patients that there is an increased risk of myocardial infarction, sudden cardiac death, and stroke with LEUPROLIDE ACETATE FOR DEPOT SUSPENSION treatment. Advise patients to immediately report signs and symptoms associated with these events to their healthcare provider for evaluation [see Warnings and Precautions (5.3)].
Urogenital Disorders
- Advise patients that LEUPROLIDE ACETATE FOR DEPOT SUSPENSION may cause impotence.
Infertility
- Inform patients that LEUPROLIDE ACETATE FOR DEPOT SUSPENSION may cause infertility [(see Use In Specific Populations (8.3)].
Continuation of LEUPROLIDE ACETATE FOR DEPOT SUSPENSION Treatment
- Inform patients that LEUPROLIDE ACETATE FOR DEPOT SUSPENSION is usually continued, often with additional medication, after the development of metastatic castration-resistant prostate cancer [see Dosage and Administration (2.1)].
For more information or a video demonstration on how to use, scan the code below or call 1-866-604-3268.

Manufactured by:
GP-PHARM, S.A.
08777 Sant Quintí de Mediona
Spain
Distributed by:
Cipla USA, Inc.
10 Independence Boulevard, Suite 300
Warren, NJ 07059
Rx only
Revised: 11/2023
21101070
NDC 69097-909-50 Rx Only
Leuprolide acetate
for depot suspension
22.5 mg
For intramuscular injection
See package insert for reconstitution and administration procedures
Recommended dose: one intramuscular injection every three month
Sterile
Must be reconstituted before use
Cipla