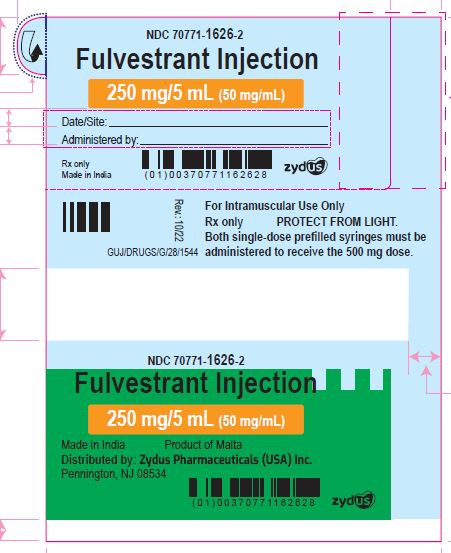
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Fulvestrant Injection
250 mg/5 mL (50 mg/mL)
For Intramuscular Use Only
Both single-dose prefilled syringes must be administered to receive the 500 mg dose.
PROTECT FROM LIGHT
Rx only
Made in India
Fulvestrant Injection
250 mg/5 mL (50 mg/mL)
For Intramuscular Use Only
This carton contains a total of 500 mg fulvestrant in TWO single-dose prefilled syringes each containing 250 mg/5 mL, and two Safety Glide™ shielding intramuscular injection needles.
Discard each syringe after use.
Both single-dose prefilled syringes must be administered to receive the 500 mg dose.
STORAGE: REFRIGERATE 2° to 8°C (36° to 46°F). TO PROTECT FROM LIGHT, STORE IN THE ORIGINAL CARTON UNTIL TIME OF USE.
Contains 2 Single-Dose Prefilled Syringes
Rx only
