Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 1/2 to 6 hours
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium restricted diet
- stomach pain, nausea or vomiting
- noticed a sudden change in bowel habits that lasts more than 2 weeks
Directions
- do not exceed more than 2 doses per day; if necessary repeat dosage in 4 hours
| age | dose |
| adults and children 12 years and older | 2-4 level teaspoons dissolved in a full glass (8oz) of water |
| children 6 to 11 years |
1-2 level teaspoons dissolved in a full glass (8oz) of water |
Not recommended for children under 6 years of age.
Other information
- each teaspoon contains: magnesium 495 mg
- store in a cool dry place out of direct sunlight
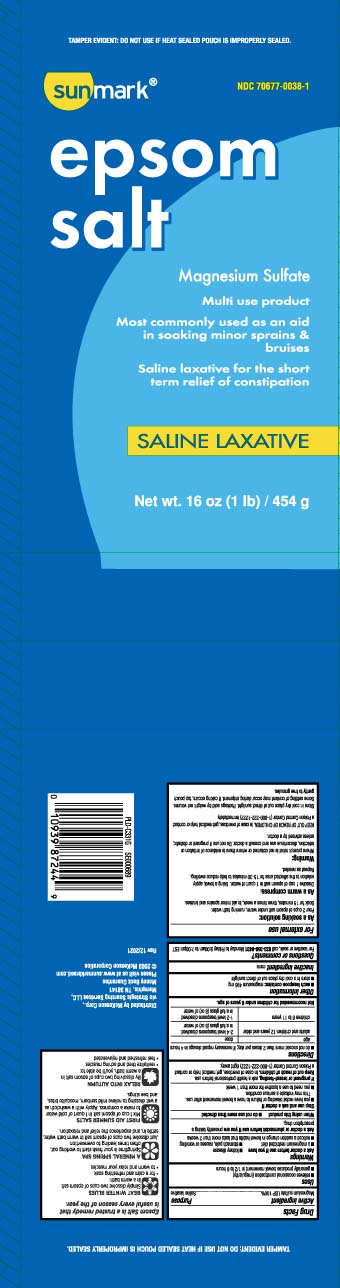
Principal Display Panel
Epsom salt
Magnesium Sulfate
Multi use product
Most commonly used as an aid
in soaking minor sprains & bruises
Saline laxative for the short term relief of constipation
SALINE LAXATIVE
Net Wt LB (kg)
*TAMPER EVIDENT: DO NOT USE IF HEAT SEALED POUCH IS IMPROPERLY SEALED.
Distributed by McKesson Corp.,
via Strategic Sourcing Services LLC,
Memphis, TN 38141