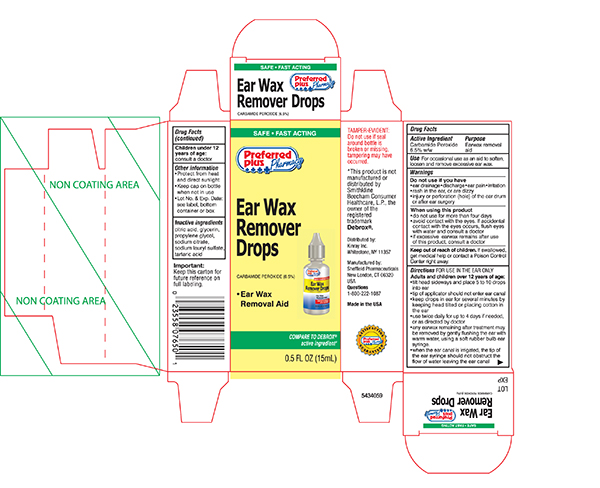
Warnings
Do not use if you have
- eardrainage, discharge, ear pain,irritation
- rashin the ear,or are dizzy
- injuryor perforation (hole) of the ear drum or after ear surgery
When using this product
- do not use for more than four days
- avoid contact with the eyes. If accidental contact with the eyes occurs, flush eyes with water and consult a doctor
- if excessive earwax remains after the use of this product, consult a doctor
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions FOR USE IN THE EAR ONLY
- Adults and children over 12 years of age:
- Tilt head sideways and place 5 to 10 drops into ear.
- Tip of applicator should not enter ear canal.
- Keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear.
- Use twice daily for up to 4 days if needed, or as directed by a doctor.
- Any earwax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe.
- When the ear canal is irrigated, the tip of the ear syringe should not obstruct the flow of water leaving the ear canal.
- Children under 12 years: consult a doctor.
Other information
- Protect from heat and direct sunlight
- Keep cap on bottle when not in use.
- Lot No. and EXP date: see label, bottom container or box.
Inactive ingredients
Citric Acid, Glycerin, Propylene Glycol, Sodium Citrate, Sodium Lauryl Sulfate, Tartaric Acid