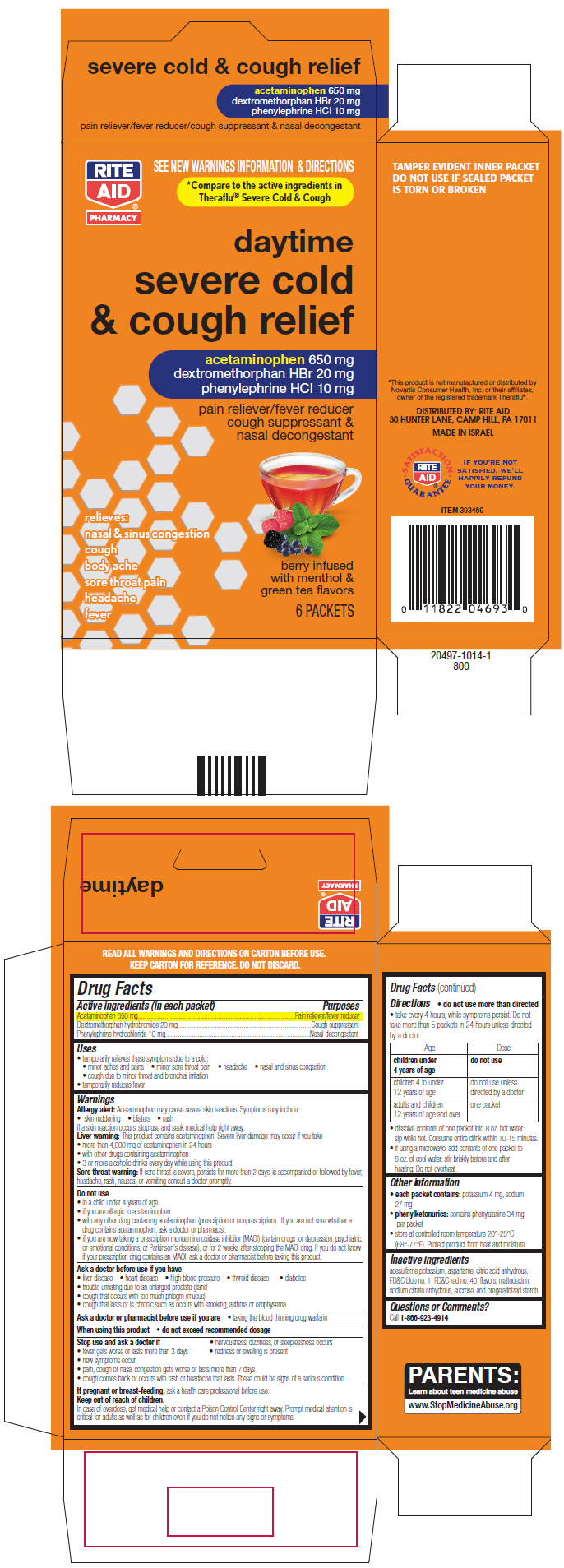
Uses
- temporarily relieves these symptoms due to a cold:
- minor aches and pains
- minor sore throat pain
- headache
- nasal and sinus congestion
- cough due to minor throat and bronchial irritation
- temporarily reduces fever
Warnings
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting consult a doctor promptly.
Do not use
- in a child under 4 years of age
- if you are allergic to acetaminophen
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occurs
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- pain, cough or nasal congestion gets worse or lasts more than 7 days
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
Directions
- do not use more than directed
- take every 4 hours, while symptoms persist. Do not take more than 5 packets in 24 hours unless directed by a doctor
| Age | Dose |
|---|---|
| children under 4 years of age | do not use |
| children 4 to under 12 years of age | do not use unless directed by a doctor |
| adults and children 12 years of age and over | one packet |
- dissolve contents of one packet into 8 oz. hot water: sip while hot. Consume entire drink within 10-15 minutes.
- if using a microwave, add contents of one packet to 8 oz. of cool water: stir briskly before and after heating. Do not overheat.
Other information
- each packet contains: potassium 4 mg, sodium 27 mg
- phenylketonurics: contains phenylalanine 34 mg per packet
- store at controlled room temperature 20°-25°C (68°-77°F). Protect product from heat and moisture.
Inactive ingredients
acesulfame potassium, aspartame, citric acid anhydrous, FD&C blue no. 1, FD&C red no. 40, flavors, maltodextrin, sodium citrate anhydrous, sucrose, and pregelatinized starch.
PRINCIPAL DISPLAY PANEL - 6 Packet Carton
RITE
AID®
PHARMACY
SEE NEW WARNINGS INFORMATION & DIRECTIONS
*Compare to the active ingredients in
Theraflu® Severe Cold & Cough
daytime
severe cold
& cough relief
acetaminophen 650 mg
dextromethorphan HBr 20 mg
phenylephrine HCl 10 mg
pain reliever/fever reducer
cough suppressant &
nasal decongestant
relieves:
nasal & sinus congestion
cough
body ache
sore throat pain
headache
fever
berry infused
with menthol &
green tea flavors
6 PACKETS