Approved by FDA under NADA # 139-237
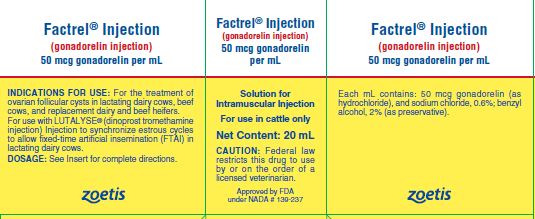
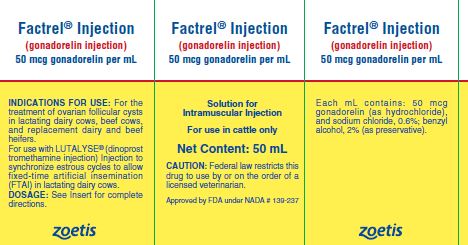
Factrel® Injection
(gonadorelin injection)
50 mcg gonadorelin per mL (as gonadorelin hydrochloride) Solution for Intramuscular Injection.
For use in cattle only
DESCRIPTION
FACTREL Injection is a sterile solution containing 50 micrograms of synthetic gonadorelin (as hydrochloride) per mL in aqueous formulation containing 0.6% sodium chloride and 2% benzyl alcohol (as a preservative).
Gonadorelin is the gonadotropin releasing hormone (GnRH) which is produced by the hypothalamus and causes the release of the gonadotropin luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary.
FACTREL Injection has the identical amino acid sequence as endogenous gonadorelin; 5-oxo Pro-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2 with identical physiological activities. The molecular weight of gonadorelin is 1182 with a molecular formula of C55H75N17O13. The corresponding values for gonadorelin hydrochloride are 1219 (1 HCl) expressed as C55H75N17O13HCl, or 1255 (2 HCl) expressed as C55H75N17O13 2HCl.
INDICATIONS FOR USE
For the treatment of ovarian follicular cysts in lactating dairy cows, beef cows, and replacement dairy and beef heifers. The treatment effect of FACTREL Injection when used in lactating dairy cows, beef cows, and replacement dairy and beef heifers is a reduction in the number of days to first estrus.
For use with LUTALYSE® (dinoprost tromethamine injection) Injection to synchronize estrous cycles to allow fixed-time artificial insemination (FTAI) in lactating dairy cows.
DOSAGE
For the treatment of ovarian follicular cysts in lactating dairy cows, beef cows, and replacement dairy and beef heifers: Administer 2 mL of FACTREL Injection as a single intramuscular injection.
For use with LUTALYSE (dinoprost tromethamine injection) Injection to synchronize estrous cycles to allow fixed-time artificial insemination (FTAI) in lactating dairy cows: Administer 2 to 4 mL FACTREL Injection (100-200 mcg gonadorelin) per cow as an intramuscular injection in a treatment regimen with the following framework:
• Administer the first dose of FACTREL Injection (2-4 mL) at Day 0
• Administer LUTALYSE (25 mg dinoprost, as dinoprost tromethamine injection) Injection by intramuscular injection 6-8 days after the first dose of FACTREL Injection.
• Administer a second dose of FACTREL Injection (2-4 mL) 30 to 72 hours after the LUTALYSE injection.
• Perform FTAI 0 to 24 hours after the second dose of FACTREL Injection, or inseminate cows on detected estrus using standard herd practices.
Below are three examples of treatment regimens for FTAI that fit within the dosage regimen framework described immediately above:
|
Example 1 |
Example 2 |
Example 3 |
|
|
Day 0 (Monday) |
1st FACTREL |
1st FACTREL |
1st FACTREL |
|
Day 7 (the following Monday) |
LUTALYSE |
LUTALYSE |
LUTALYSE |
|
Day 9 (Wednesday) |
2nd FACTREL + FTAI at 48 hours after LUTALYSE |
2nd FACTREL 48 hours after LUTALYSE |
2nd FACTREL 56 hours after LUTALYSE |
|
Day 10 (Thursday) |
FTAI 24 hours after 2nd FACTREL |
FTAI 18 hours after 2nd FACTREL |
MECHANISM OF ACTION
Follicular cysts are enlarged non-ovulatory follicles resulting from a malfunction of the neuroendocrine mechanism controlling follicular maturation and ovulation. Exogenous administration of agents possessing luteinizing hormone (LH) activity, such as pituitary extracts or human chorionic gonadotropin, often causes ovulation or regression of follicular cysts. FACTREL Injection induces release of endogenous luteinizing hormone (LH) to produce this same effect.
Gonadorelin, through release of LH has been demonstrated to induce ovulation of dominant ovarian follicles present on the bovine ovary during the estrous cycle. Administration of FACTREL Injection has the same effect.
RESIDUE WARNINGS
No withdrawal period or milk discard time is required when used according to labeling.
EFFECTIVENESS
For the treatment of ovarian follicular cysts in lactating dairy cows, beef cows, and replacement dairy and beef heifers:
The treatment effect of FACTREL Injection when used in lactating dairy cows, beef cows, and replacement dairy and beef heifers is a reduction in the number of days to first estrus.
There were no significant differences in days from treatment to conception, frequency of cows conceiving at first or subsequent heats, or conception rates among treated or non-treated control animals, when FACTREL Injection was used alone for treatment of cystic ovaries.
For use with LUTALYSE (dinoprost tromethamine injection) Injection to synchronize estrous cycles to allow fixed-time artificial insemination (FTAI) in lactating dairy cows:
A field study was conducted to compare control (0 mL FACTREL Injection) to two doses of 2, 3 or 4 mL FACTREL Injection (100-200 mcg gonadorelin) for use with LUTALYSE Injection to synchronize estrous cycles to allow FTAI in lactating dairy cows under field conditions. Cows were examined prior to study start and only clinically normal cows were enrolled. A total of 1142 cows were enrolled at 6 commercial dairies. Cows were assigned randomly in blocks of 4 cows to each of 4 treatment groups consisting of:
Day 0: 2, 3 or 4 mL dose of FACTREL Injection or no injection (Control)
Day 7: 5 mL LUTALYSE Injection (all treatment groups)
Day 9: 2, 3 or 4 mL dose of FACTREL Injection or no injection (Control)
Day 10: Fixed-time artificial insemination
On Day 9 the second dose of FACTREL Injection (cows received the same dose as for first treatment) was given either 48 or 56 hours after the dose of LUTALYSE Injection and FTAI was conducted 24 or 17 hours later, respectively. For control cows FTAI was performed 72 hours after the LUTALYSE Injection dose was administered. All treatment groups had significantly greater pregnancy rates to FTAI than cows administered LUTALYSE Injection alone, and were 17.1, 27.3, 29.1 and 32.2% for cows receiving 0 (Control), 2, 3 or 4 mL FACTREL Injection, respectively.
SAFETY AND TOXICITY
In cows the intramuscular administration of up to 12.5 times maximum recommended dosage (2,500 mcg/day) of FACTREL Injection for 3 days did not affect any physiological or clinical parameter. Likewise, single intramuscular doses of 500 mcg did not interfere with pregnancy. No evidence of irritation at injection site was found in any animal.
A total of 1142 cows were enrolled in the previously noted field study that evaluated the effectiveness of two doses of 2, 3 or 4 mL of FACTREL Injection for use with LUTALYSE Injection to synchronize estrous cycles to allow FTAI in lactating dairy cows. Cows were observed daily for abnormal clinical signs. Over the course of the study there were 148 adverse health events documented in 118 cows. These adverse health events were common conditions in dairy cows (mastitis, lameness and pneumonia) and are not considered related to treatment.
CONTACT INFORMATION
For a copy of the Safety Data Sheet or to report adverse reactions, call Zoetis Inc. at 1-888-963-8471.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
HOW SUPPLIED
FACTREL Injection (gonadorelin injection), 50 mcg/mL is available in 20 mL and 50 mL multi-dose vials (box of one).
STORAGE CONDITIONS
Store at refrigerator temperature 2° to 8°C (36° to 46°F), with excursions permitted to 25°C (77°F). Use contents within 1 month of first vial puncture.