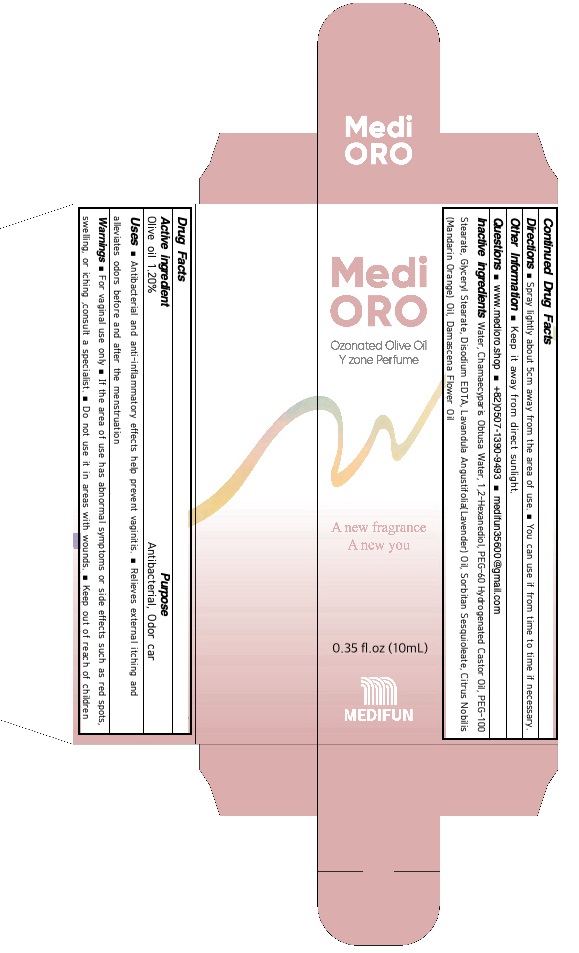
Uses
■ Antibacterial and anti-inflammatory effects help prevent vaginitis.
■ Relieves external itching and alleviates odors before and after the menstruation
WARNINGS
For vaginal use only
If the area of use has abnormal symptoms or side effects such as red spots, swelling, or iching, consult a specialist.
Do not use it in areas with wounds.
Don’t use it right after removing the fur.
Keep out of reach of children
Directions
■ Spray lightly about 5cm away from the area of use.
■ You can use if from time to time if necessary.
Other Information
■ Do not store this product in an inappropriate place such as high or low temperatures or under direct sun light