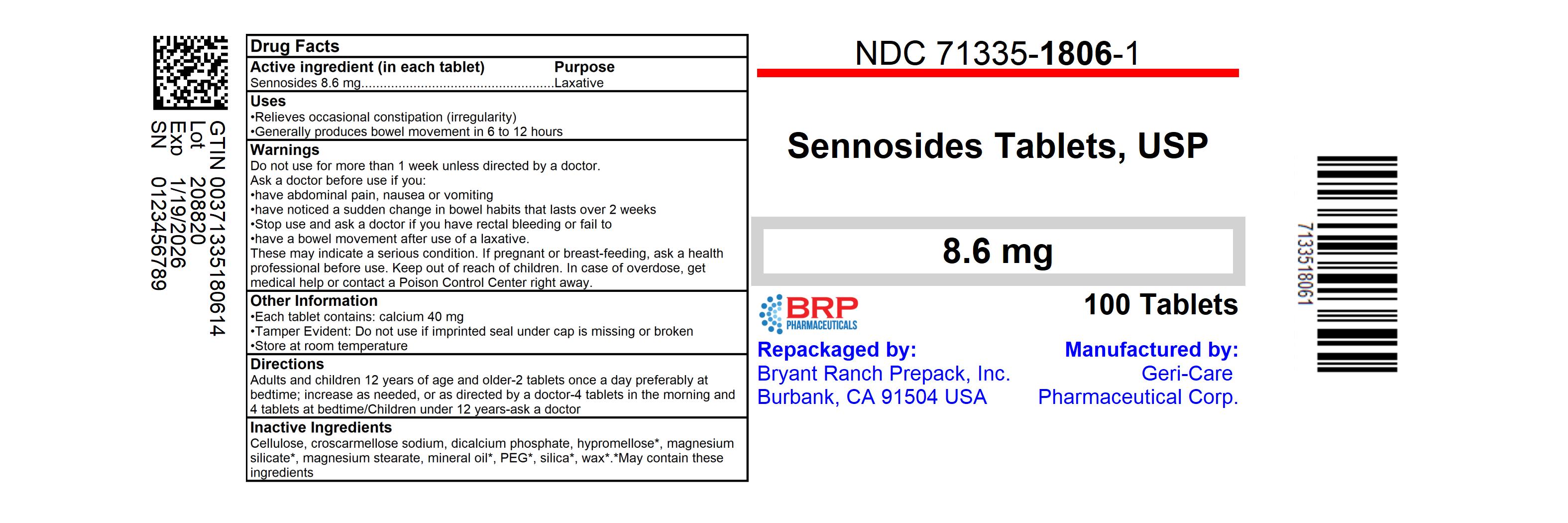
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6 to 12 hours
Warnings
Do not use
for more than 1 week unless directed by a doctor.
Ask a doctor before use if you
- have abdominal pain, nausea or vomiting
- have noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to
have a bowel movement after use of a laxative.
These may indicate a serious condition
.
If pregnant or breast-feeding,
ask a health professional before use.
Directions
• do not exceed 8 tablets in 24 hours
| Age
| Starting Dose
| Maximum Dose
|
|---|---|---|
| adults and children 12 years of age and older
| 2 tablets once a day preferably at bedtime; increase as needed, or as directed by a doctor
| 4 tablets in the morning and 4 tablets at bedtime
|
| children under 12 years
| ask a doctor
|
Other information
- each tablet contains: calcium 40 mg
- Tamper Evident: Do not use if imprinted seal under cap is missing or broken
- store at room temperature
Inactive ingredients
cellulose, croscarmellose sodium,
dicalcium phosphate, hypromellose*, magnesium silicate*,
magnesium stearate, mineral oil*, PEG*, silica*, wax*.
*May contain these ingredients
HOW SUPPLIED
NDC: 71335-1806-1: 100 Tablets in a BOTTLE
NDC: 71335-1806-2: 60 Tablets in a BOTTLE
NDC: 71335-1806-3: 120 Tablets in a BOTTLE
NDC: 71335-1806-4: 56 Tablets in a BOTTLE
NDC: 71335-1806-5: 30 Tablets in a BOTTLE
NDC: 71335-1806-6: 90 Tablets in a BOTTLE
NDC: 71335-1806-7: 10 Tablets in a BOTTLE
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504