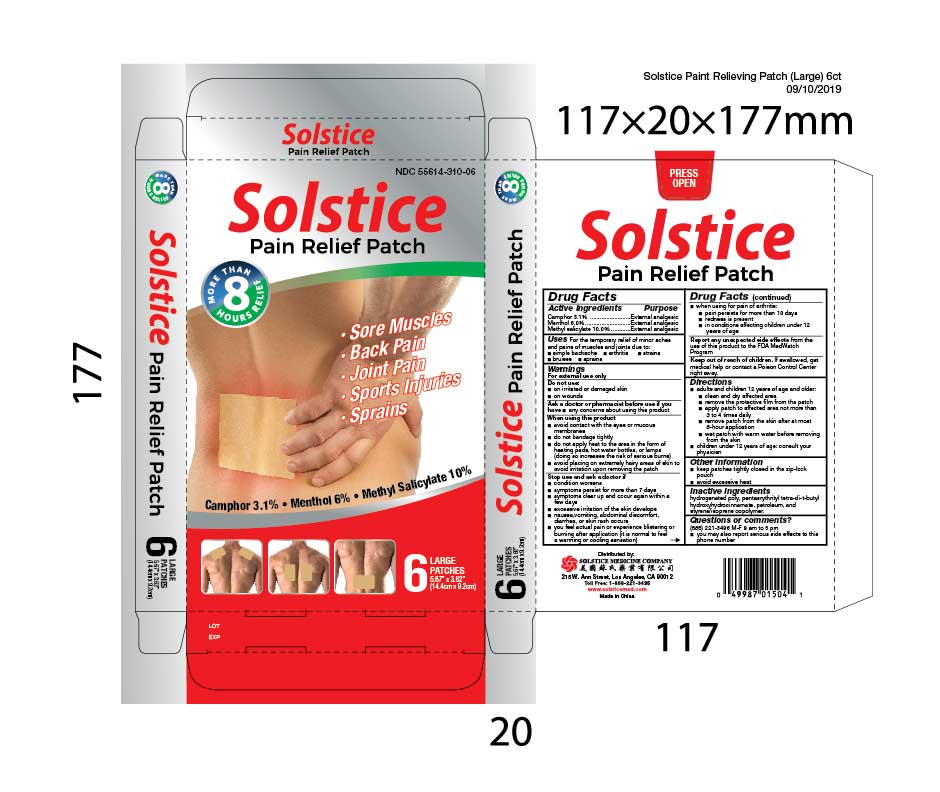
Uses
For the temporary relief of minor aches and pains of muscles and joints due to:
- simple backache
- arthritis
- strains
- bruises
- sprains
When using this product
- avoid contact with the eyes or mucous membranes
- do not bandage tightly
- do not apply heat to the area in the form of heating pads, hot water bottles, or lamps (doing so increases the risk of serious burns)
- avoid placing on extremely hairy areas of skin to avoid irritation upon removing the patch
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive irritation of the skin develops
- nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
- you feel actual pain or experience blistering or burning after application (it is normal to feel a warming or cooling sensation)
- when using for pain of arthritis:
- pain persists for more than 10 days
- redness is present
- in conditions affecting children under 12 years of age
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 3 years of age and older:
- clean and dry affected area
- remove the protective film from the patch
- apply patch to affected area not more than 3 to 4 times daily
- remove patch from the skin after at most 8-hour application
- wet patch with warm water before removing from the skin
- children under 3 years of age: consult your physician
Inactive ingredients hydrogenated poly, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, petroleum, and styrene/isoprene copolymer.