FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adult Patients
PREZISTA®, co-administered with ritonavir (PREZISTA/ritonavir), and with other antiretroviral agents, is indicated for the treatment of human immunodeficiency virus (HIV-1) infection.
This indication is based on analyses of plasma HIV-1 RNA levels and CD4+ cell counts from 2 controlled Phase 3 trials of 48 weeks duration in antiretroviral treatment-naïve and treatment-experienced patients and 2 controlled Phase 2 trials of 96 weeks duration in clinically advanced, treatment-experienced adult patients.
1.2 Pediatric Patients
PREZISTA, co-administered with ritonavir (PREZISTA/ritonavir), and with other antiretroviral agents, is indicated for the treatment of HIV-1 infection in pediatric patients 3 years of age and older [see Use in Specific Populations (8.4)].
This indication is based on 24-week analyses of plasma HIV-1 RNA levels and CD4+ cell counts from 2 open-label Phase 2 trials in antiretroviral treatment-experienced pediatric patients (one trial in patients 6 to less than 18 years of age and one trial in patients 3 to less than 6 years of age).
In treatment-experienced adult and pediatric patients, the following points should be considered when initiating therapy with PREZISTA/ritonavir:
- Treatment history and, when available, genotypic or phenotypic testing should guide the use of PREZISTA/ritonavir [see Clinical Pharmacology (12.4)].
- The use of other active agents with PREZISTA/ritonavir is associated with a greater likelihood of treatment response [see Clinical Pharmacology (12.4) and Clinical Studies (14.3)].
2 DOSAGE AND ADMINISTRATION
2.1 Adult Patients
PREZISTA must be co-administered with ritonavir to exert its therapeutic effect. Failure to correctly co-administer PREZISTA with ritonavir will result in plasma levels of darunavir that will be insufficient to achieve the desired antiviral effect and will alter some drug interactions.
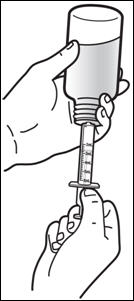
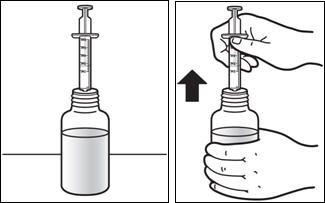
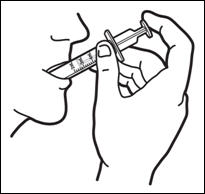
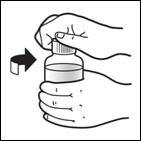
Patients who have difficulty swallowing PREZISTA tablets can use the 100 mg/mL PREZISTA oral suspension.
Treatment-Naïve Adult Patients
The recommended oral dose of PREZISTA is 800 mg (two 400 mg tablets or 8 mL of the oral suspension) taken with ritonavir 100 mg (one 100 mg tablet/capsule or 1.25 mL of a 80 mg/mL ritonavir oral solution) once daily and with food.
Treatment-Experienced Adult Patients
| Treatment-Experienced Adult Patients | |
|---|---|
|
|
| With no darunavir resistance associated substitutions* | With at least one darunavir resistance associated substitution* |
| PREZISTA 800 mg (two 400 mg tablets or 8 mL) once daily with ritonavir 100 mg (one 100 mg tablet/capsule or 1.25 mL) once daily and with food | PREZISTA 600 mg (e.g. one 600 mg tablet or 6 mL) twice daily with ritonavir 100 mg (one 100 mg tablet/capsule or 1.25 mL) twice daily and with food |
For antiretroviral treatment-experienced patients genotypic testing is recommended. However, when genotypic testing is not feasible, PREZISTA/ritonavir 600/100 mg twice daily dosing is recommended.
2.2 Pediatric Patients (age 3 to less than 18 years)
Do not use once daily dosing in pediatric patients.
Healthcare professionals should pay special attention to accurate dose selection of PREZISTA, transcription of the medication order, dispensing information and dosing instruction to minimize risk for medication errors, overdose, and underdose.
Prescribers should select the appropriate dose of PREZISTA/ritonavir for each individual child based on body weight (kg) and should not exceed the recommended dose for treatment-experienced adults.
Before prescribing PREZISTA, children weighing greater than or equal to 15 kg should be assessed for the ability to swallow tablets. If a child is unable to reliably swallow a tablet, the use of PREZISTA oral suspension should be considered.
The recommended dose of PREZISTA/ritonavir for pediatric patients (3 to less than 18 years of age and weighing at least 10 kg is based on body weight (see Tables 1, 2 and 3) and should not exceed the recommended treatment-experienced adult dose (PREZISTA/ritonavir 600/100 mg twice daily). PREZISTA should be taken with ritonavir twice daily and with food.
Dosing recommendations for pediatric patients weighing at least 10 kg but less than 15 kg
The weight-based dose in pediatric patients weighing less than 15 kg is PREZISTA 20 mg/kg with ritonavir 3 mg/kg which can be dosed using the following table:
| Body weight (kg) | Dose (twice daily with food) |
|---|---|
| Greater than or equal to 10 kg to less than 11 kg | PREZISTA 200 mg (2 mL) with ritonavir 32 mg (0.4 mL) |
| Greater than or equal to 11 kg to less than 12 kg | PREZISTA 220 mg (2.2 mL) with ritonavir 32 mg (0.4 mL) |
| Greater than or equal to 12 kg to less than 13 kg | PREZISTA 240 mg (2.4 mL) with ritonavir 40 mg (0.5 mL) |
| Greater than or equal to 13 kg to less than 14 kg | PREZISTA 260 mg (2.6 mL) with ritonavir 40 mg (0.5 mL) |
| Greater than or equal to 14 kg to less than 15 kg | PREZISTA 280 mg (2.8 mL) with ritonavir 48 mg (0.6 mL) |
Dosing recommendations for pediatric patients weighing at least 15 kg
Pediatric patients who weigh at least 15 kg and are able to swallow tablets can be dosed using the following table:
| Body Weight (kg) | Dose (twice daily with food) |
|---|---|
|
|
| Greater than or equal to 15 kg to less than 30 kg | PREZISTA 375 mg with ritonavir* 50 mg (0.6 mL) |
| Greater than or equal to 30 kg to less than 40 kg | PREZISTA 450 mg with ritonavir* 60 mg (0.75 mL) |
| Greater than or equal to 40 kg | PREZISTA 600 mg with ritonavir100 mg |
Pediatric patients who weigh at least 15 kg but are unable to swallow tablets can be dosed using the following table:
| Body Weight (kg) | Dose (twice daily with food) |
|---|---|
| Greater than or equal to 15 kg to less than 30 kg | PREZISTA 375 mg (3.8 mL) with ritonavir 50 mg (0.6 mL) |
| Greater than or equal to 30 kg to less than 40 kg | PREZISTA 450 mg (4.6 mL) with ritonavir 60 mg (0.75 mL) |
| Greater than or equal to 40 kg | PREZISTA 600 mg (6 mL) with ritonavir 100 mg (1.25 mL) |
Do not use PREZISTA/ritonavir in pediatric patients below 3 years of age [see Warnings and Precautions (5.11) and Nonclinical Toxicology (13.2)].
2.3 Patients with Hepatic Impairment
No dose adjustment is required in patients with mild or moderate hepatic impairment. No data are available regarding the use of PREZISTA/ritonavir when co-administered to subjects with severe hepatic impairment; therefore, PREZISTA/ritonavir is not recommended for use in patients with severe hepatic impairment [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
3.1 PREZISTA 100 mg/mL Oral Suspension
PREZISTA (darunavir) 100 mg/mL oral suspension is supplied as a white to off-white opaque suspension for oral use, containing darunavir ethanolate equivalent to 100 mg of darunavir per mL of suspension.
3.2 PREZISTA 75 mg Tablets
PREZISTA (darunavir) 75 mg tablets are supplied as white, caplet-shaped, film-coated tablets containing darunavir ethanolate equivalent to 75 mg of darunavir per tablet. Each tablet is debossed with "75" on one side and "TMC" on the other side.
3.3 PREZISTA 150 mg Tablets
PREZISTA (darunavir) 150 mg tablets are supplied as white, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 150 mg of darunavir per tablet. Each tablet is debossed with "150" on one side and "TMC" on the other side.
4 CONTRAINDICATIONS
Co-administration of PREZISTA/ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). These drugs and other contraindicated drugs (which may lead to reduced efficacy of darunavir) are listed in Table 4 [also see Drug Interactions (7.3), Table 9].
| Drug Class | Drugs Within Class That Are Contraindicated With PREZISTA/ritonavir | Clinical Comment |
|---|---|---|
| Alpha 1-adrenoreceptor antagonist | Alfuzosin | Potential for serious and/or life-threatening reactions such as hypotension. |
| Ergot Derivatives | Dihydroergotamine, Ergonovine, Ergotamine, Methylergonovine | Potential for serious and/or life-threatening events such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. |
| GI Motility Agent | Cisapride | Potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
| Neuroleptic | Pimozide | Potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
| Sedative/hypnotics | Orally administered Midazolam, Triazolam | Triazolam and orally administered midazolam are extensively metabolized by CYP3A. Co-administration of triazolam or orally administered midazolam with PREZISTA/ritonavir may cause large increases in the concentrations of these benzodiazepines. Potential for serious and/or life-threatening events such as prolonged or increased sedation or respiratory depression. |
| Herbal Products | St. John's Wort (Hypericum perforatum) | Patients taking PREZISTA/ritonavir should not use products containing St. John's wort because co-administration may result in reduced plasma concentrations of darunavir. This may result in loss of therapeutic effect and development of resistance. |
| HMG-CoA Reductase Inhibitors | Lovastatin, Simvastatin | Potential for serious reactions such as myopathy including rhabdomyolysis. For dosing recommendation regarding atorvastatin and pravastatin, see Table 9: Established and Other Potentially Significant Drug Interactions: Alterations in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interaction. |
| Antimycobacterial | Rifampin | Rifampin is a potent inducer of CYP450 metabolism. PREZISTA/ritonavir should not be used in combination with rifampin, as this may cause significant decreases in darunavir plasma concentrations. This may result in loss of therapeutic effect to PREZISTA. |
| PDE-5 inhibitor | Sildenafil for treatment of pulmonary arterial hypertension | A safe and effective dose for the treatment of pulmonary arterial hypertension has not been established with PREZISTA/ritonavir. There is an increased potential for sildenafil-associated adverse events (which include visual disturbances, hypotension, prolonged erection, and syncope). |
Due to the need for co-administration of PREZISTA with ritonavir, please refer to ritonavir prescribing information for a description of ritonavir contraindications.
5 WARNINGS AND PRECAUTIONS
5.1 General
PREZISTA must be co-administered with ritonavir and food to achieve the desired antiviral effect. Failure to administer PREZISTA with ritonavir and food may result in a loss of efficacy of darunavir.
Please refer to ritonavir prescribing information for additional information on precautionary measures.
5.2 Hepatotoxicity
Drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) has been reported with PREZISTA/ritonavir. During the clinical development program (N=3063), hepatitis was reported in 0.5% of patients receiving combination therapy with PREZISTA/ritonavir. Patients with pre-existing liver dysfunction, including chronic active hepatitis B or C, have an increased risk for liver function abnormalities including severe hepatic adverse events.
Post-marketing cases of liver injury, including some fatalities, have been reported. These have generally occurred in patients with advanced HIV-1 disease taking multiple concomitant medications, having co-morbidities including hepatitis B or C co-infection, and/or developing immune reconstitution syndrome. A causal relationship with PREZISTA/ritonavir therapy has not been established.
Appropriate laboratory testing should be conducted prior to initiating therapy with PREZISTA/ritonavir and patients should be monitored during treatment. Increased AST/ALT monitoring should be considered in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases, especially during the first several months of PREZISTA/ritonavir treatment.
Evidence of new or worsening liver dysfunction (including clinically significant elevation of liver enzymes and/or symptoms such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, hepatomegaly) in patients on PREZISTA/ritonavir should prompt consideration of interruption or discontinuation of treatment.
5.3 Severe Skin Reactions
During the clinical development program (n=3063), severe skin reactions, accompanied by fever and/or elevations of transaminases in some cases, have been reported in 0.4% of subjects. Stevens-Johnson Syndrome was rarely (less than 0.1%) reported during the clinical development program. During post-marketing experience toxic epidermal necrolysis and acute generalized exanthematous pustulosis have been reported. Discontinue PREZISTA/ritonavir immediately if signs or symptoms of severe skin reactions develop. These can include but are not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis and/or eosinophilia.
Rash (all grades, regardless of causality) occurred in 10.3% of subjects treated with PREZISTA/ritonavir [also see Adverse Reactions (6)]. Rash was mostly mild-to-moderate, often occurring within the first four weeks of treatment and resolving with continued dosing. The discontinuation rate due to rash in subjects using PREZISTA/ritonavir was 0.5%.
Rash occurred more commonly in treatment-experienced subjects receiving regimens containing PREZISTA/ritonavir + raltegravir compared to subjects receiving PREZISTA/ritonavir without raltegravir or raltegravir without PREZISTA/ritonavir. However, rash that was considered drug related occurred at similar rates for all three groups. These rashes were mild to moderate in severity and did not limit therapy; there were no discontinuations due to rash.
5.4 Sulfa Allergy
Darunavir contains a sulfonamide moiety. PREZISTA should be used with caution in patients with a known sulfonamide allergy. In clinical studies with PREZISTA/ritonavir, the incidence and severity of rash were similar in subjects with or without a history of sulfonamide allergy.
5.5 Drug Interactions
See Table 4 for a listing of drugs that are contraindicated for use with PREZISTA/ritonavir due to potentially life-threatening adverse events, significant drug-drug interactions, or loss of therapeutic effect to PREZISTA [see Contraindications (4)]. Please refer to Table 9 for established and other potentially significant drug-drug interactions [see Drug Interactions (7.3)].
5.6 Diabetes Mellitus / Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease inhibitor (PI) therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued PI therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and causal relationships between PI therapy and these events have not been established.
5.7 Fat Redistribution
Redistribution/accumulation of body fat, including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.8 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including PREZISTA. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of antiretroviral treatment.
5.9 Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis in patients with hemophilia type A and B treated with PIs. In some patients, additional factor VIII was given. In more than half of the reported cases, treatment with PIs was continued or reintroduced if treatment had been discontinued. A causal relationship between PI therapy and these episodes has not been established.
5.10 Resistance/Cross-Resistance
Because the potential for HIV cross-resistance among PIs has not been fully explored in PREZISTA/ritonavir treated patients, the effect therapy with PREZISTA will have on the activity of subsequently administered PIs is unknown [see Microbiology (12.4)].
5.11 Pediatric Patients
Do not administer PREZISTA/ritonavir in pediatric patients below 3 years of age in view of toxicity and mortality observed in juvenile rats dosed with darunavir (from 20 mg/kg to 1000 mg/kg) up to days 23 to 26 of age [see Use in Specific Populations (8.1 and 8.4), Clinical Pharmacology (12.3), and Nonclinical Toxicology (13.2)].
6 ADVERSE REACTIONS
The overall safety profile of PREZISTA/ritonavir 800/100 mg once daily and PREZISTA/ritonavir 600/100 mg twice daily is based on clinical trials and post-marketing data, and is consistent with the data presented below.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Due to the need for co-administration of PREZISTA with ritonavir, please refer to ritonavir prescribing information for ritonavir-associated adverse reactions.
6.1 Clinical Trials Experience: Treatment-Naïve Adults
Study TMC114-C211
The safety assessment is based on all safety data from the Phase 3 trial TMC114-C211 comparing PREZISTA/ritonavir 800/100 mg once daily versus lopinavir/ritonavir 800/200 mg per day in 689 antiretroviral treatment-naïve HIV-1-infected adult subjects. The total mean exposure for subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and in the lopinavir/ritonavir 800/200 mg per day arm was 162.5 and 153.5 weeks, respectively.
The majority of the adverse drug reactions (ADRs) reported during treatment with PREZISTA/ritonavir 800/100 mg once daily were mild in severity. The most common clinical ADRs to PREZISTA/ritonavir 800/100 mg once daily (greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, headache, abdominal pain and rash. 2.3% of subjects in the PREZISTA/ritonavir arm discontinued treatment due to ADRs.
ADRs to PREZISTA/ritonavir 800/100 mg once daily of at least moderate intensity (greater than or equal to Grade 2) in antiretroviral treatment-naïve HIV-1-infected adult subjects are presented in Table 5 and subsequent text below the table.
| Randomized Study TMC114-C211 |
||
|---|---|---|
| System Organ Class, Preferred Term, % | PREZISTA/ritonavir 800/100 mg once daily + TDF/FTC N = 343 | lopinavir/ritonavir 800/200 mg per day + TDF/FTC N = 346 |
| N=total number of subjects per treatment group TDF = tenofovir disoproxil fumarate FTC = emtricitabine |
||
| Gastrointestinal Disorders | ||
| Abdominal pain | 6% | 6% |
| Diarrhea | 9% | 16% |
| Nausea | 4% | 4% |
| Vomiting | 2% | 4% |
| General Disorders and Administration Site Conditions | ||
| Fatigue | < 1% | 3% |
| Metabolism and Nutrition Disorders | ||
| Anorexia | 2% | < 1% |
| Nervous System Disorders | ||
| Headache | 7% | 6% |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash | 6% | 7% |
Less Common Adverse Reactions
Treatment-emergent ADRs of at least moderate intensity (greater than or equal to Grade 2) occurring in less than 2% of antiretroviral treatment-naïve subjects receiving PREZISTA/ritonavir 800/100 mg once daily are listed below by body system:
Gastrointestinal Disorders: acute pancreatitis, dyspepsia, flatulence
General Disorders and Administration Site Conditions: asthenia
Hepatobiliary Disorders: acute hepatitis (e.g., acute hepatitis, cytolytic hepatitis, hepatotoxicity)
Immune System Disorders: (drug) hypersensitivity, immune reconstitution syndrome
Metabolism and Nutrition Disorders: diabetes mellitus
Musculoskeletal and Connective Tissue Disorders: myalgia, osteonecrosis
Psychiatric Disorders: abnormal dreams
Skin and Subcutaneous Tissue Disorders: angioedema, pruritus, Stevens-Johnson Syndrome, urticaria
Laboratory abnormalities:
Selected Grade 2 to 4 laboratory abnormalities that represent a worsening from baseline observed in antiretroviral treatment-naïve adult subjects treated with PREZISTA/ritonavir 800/100 mg once daily are presented in Table 6.
| Randomized Study TMC114-C211 |
|||
|---|---|---|---|
| Laboratory Parameter Preferred Term, % | Limit | PREZISTA/ritonavir 800/100 mg once daily + TDF/FTC | lopinavir/ritonavir 800/200 mg per day + TDF/FTC |
| N=total number of subjects per treatment group TDF = tenofovir disoproxil fumarate FTC = emtricitabine |
|||
| Biochemistry | |||
| Alanine Aminotransferase | |||
| Grade 2 | > 2.5 to ≤ 5.0 × ULN | 9% | 9% |
| Grade 3 | > 5.0 to ≤ 10.0 × ULN | 3% | 3% |
| Grade 4 | > 10.0 × ULN | < 1% | 3% |
| Aspartate Aminotransferase | |||
| Grade 2 | > 2.5 to ≤ 5.0 × ULN | 7% | 10% |
| Grade 3 | > 5.0 to ≤ 10.0 × ULN | 4% | 2% |
| Grade 4 | > 10.0 × ULN | 1% | 3% |
| Alkaline Phosphatase | |||
| Grade 2 | > 2.5 to ≤ 5.0 × ULN | 1% | 1% |
| Grade 3 | > 5.0 to ≤ 10.0 × ULN | 0% | < 1% |
| Grade 4 | > 10.0 × ULN | 0% | 0% |
| Hyperbilirubinemia | |||
| Grade 2 | > 1.5 to ≤ 2.5 × ULN | < 1% | 5% |
| Grade 3 | > 2.5 to ≤ 5.0 × ULN | < 1% | < 1% |
| Grade 4 | > 5.0 × ULN | 0% | 0% |
| Triglycerides | |||
| Grade 2 | 5.65–8.48 mmol/L 500–750 mg/dL | 3% | 10% |
| Grade 3 | 8.49–13.56 mmol/L 751–1200 mg/dL | 2% | 5% |
| Grade 4 | > 13.56 mmol/L > 1200 mg/dL | 1% | 1% |
| Total Cholesterol | |||
| Grade 2 | 6.20–7.77 mmol/L 240–300 mg/dL | 23% | 27% |
| Grade 3 | > 7.77 mmol/L > 300 mg/dL | 1% | 5% |
| Low-Density Lipoprotein Cholesterol | |||
| Grade 2 | 4.13–4.90 mmol/L 160–190 mg/dL | 14% | 12% |
| Grade 3 | ≥ 4.91 mmol/L ≥ 191 mg/dL | 9% | 6% |
| Elevated Glucose Levels | |||
| Grade 2 | 6.95–13.88 mmol/L 126–250 mg/dL | 11% | 10% |
| Grade 3 | 13.89–27.75 mmol/L 251–500 mg/dL | 1% | <1% |
| Grade 4 | > 27.75 mmol/L > 500 mg/dL | 0% | 0% |
| Pancreatic Lipase | |||
| Grade 2 | > 1.5 to ≤ 3.0 × ULN | 3% | 2% |
| Grade 3 | > 3.0 to ≤ 5.0 × ULN | < 1% | 1% |
| Grade 4 | > 5.0 × ULN | 0% | < 1% |
| Pancreatic Amylase | |||
| Grade 2 | > 1.5 to ≤ 2.0 × ULN | 5% | 2% |
| Grade 3 | > 2.0 to ≤ 5.0 × ULN | 5% | 4% |
| Grade 4 | > 5.0 × ULN | 0% | < 1% |
6.2 Clinical Trials Experience: Treatment-Experienced Adults
Study TMC114-C214
The safety assessment is based on all safety data from the Phase 3 trial TMC114-C214 comparing PREZISTA/ritonavir 600/100 mg twice daily versus lopinavir/ritonavir 400/100 mg twice daily in 595 antiretroviral treatment-experienced HIV-1-infected adult subjects. The total mean exposure for subjects in the PREZISTA/ritonavir 600/100 mg twice daily arm and in the lopinavir/ritonavir 400/100 mg twice daily arm was 80.7 and 76.4 weeks, respectively.
The majority of the ADRs reported during treatment with PREZISTA/ritonavir 600/100 mg twice daily were mild in severity. The most common clinical ADRs to PREZISTA/ritonavir 600/100 mg twice daily (greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, nausea, rash, abdominal pain and vomiting. 4.7% of subjects in the PREZISTA/ritonavir arm discontinued treatment due to ADRs.
ADRs to PREZISTA/ritonavir 600/100 mg twice daily of at least moderate intensity (greater than or equal to Grade 2) in antiretroviral treatment-experienced HIV-1-infected adult subjects are presented in Table 7 and subsequent text below the table.
| Randomized Study TMC114-C214 |
||
|---|---|---|
| System Organ Class, Preferred Term, % | PREZISTA/ritonavir 600/100 mg twice daily + OBR N = 298 | lopinavir/ritonavir 400/100 mg twice daily + OBR N = 297 |
| N=total number of subjects per treatment group OBR = optimized background regimen |
||
| Gastrointestinal Disorders | ||
| Abdominal distension | 2% | < 1% |
| Abdominal pain | 6% | 3% |
| Diarrhea | 14% | 20% |
| Dyspepsia | 2% | 1% |
| Nausea | 7% | 6% |
| Vomiting | 5% | 3% |
| General Disorders and Administration Site Conditions | ||
| Asthenia | 3% | 1% |
| Fatigue | 2% | 1% |
| Metabolism and Nutrition Disorders | ||
| Anorexia | 2% | 2% |
| Diabetes mellitus | 2% | < 1% |
| Nervous System Disorders | ||
| Headache | 3% | 3% |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash | 7% | 3% |
Less Common Adverse Reactions
Treatment-emergent ADRs of at least moderate intensity (greater than or equal to Grade 2) occurring in less than 2% of antiretroviral treatment-experienced subjects receiving PREZISTA/ritonavir 600/100 mg twice daily are listed below by body system:
Gastrointestinal Disorders: acute pancreatitis, flatulence
Musculoskeletal and Connective Tissue Disorders: myalgia
Psychiatric Disorders: abnormal dreams
Skin and Subcutaneous Tissue Disorders: pruritus, urticaria
Laboratory abnormalities:
Selected Grade 2 to 4 laboratory abnormalities that represent a worsening from baseline observed in antiretroviral treatment-experienced adult subjects treated with PREZISTA/ritonavir 600/100 mg twice daily are presented in Table 8.
| Randomized Study TMC114-C214 |
|||
|---|---|---|---|
| Laboratory Parameter Preferred Term, % | Limit | PREZISTA/ritonavir 600/100 mg twice daily + OBR | lopinavir/ritonavir 400/100 mg twice daily + OBR |
| N=total number of subjects per treatment group OBR = optimized background regimen |
|||
|
|||
| Biochemistry | |||
| Alanine Aminotransferase | |||
| Grade 2 | > 2.5 to ≤ 5.0 × ULN | 7% | 5% |
| Grade 3 | > 5.0 to ≤ 10.0 × ULN | 2% | 2% |
| Grade 4 | > 10.0 × ULN | 1% | 2% |
| Aspartate Aminotransferase | |||
| Grade 2 | > 2.5 to ≤ 5.0 × ULN | 6% | 6% |
| Grade 3 | > 5.0 to ≤ 10.0 × ULN | 2% | 2% |
| Grade 4 | > 10.0 × ULN | < 1% | 2% |
| Alkaline Phosphatase | |||
| Grade 2 | > 2.5 to ≤ 5.0 × ULN | < 1% | 0% |
| Grade 3 | > 5.0 to ≤ 10.0 × ULN | < 1% | < 1% |
| Grade 4 | > 10.0 × ULN | 0% | 0% |
| Hyperbilirubinemia | |||
| Grade 2 | > 1.5 to ≤ 2.5 × ULN | < 1% | 2% |
| Grade 3 | > 2.5 to ≤ 5.0 × ULN | < 1% | < 1% |
| Grade 4 | > 5.0 × ULN | < 1% | 0% |
| Triglycerides | |||
| Grade 2 | 5.65–8.48 mmol/L 500–750 mg/dL | 10% | 11% |
| Grade 3 | 8.49–13.56 mmol/L 751–1200 mg/dL | 7% | 10% |
| Grade 4 | > 13.56 mmol/L > 1200 mg/dL | 3% | 6% |
| Total Cholesterol | |||
| Grade 2 | 6.20–7.77 mmol/L 240–300 mg/dL | 25% | 23% |
| Grade 3 | > 7.77 mmol/L > 300 mg/dL | 10% | 14% |
| Low-Density Lipoprotein Cholesterol | |||
| Grade 2 | 4.13–4.90 mmol/L 160–190 mg/dL | 14% | 14% |
| Grade 3 | ≥ 4.91 mmol/L ≥ 191 mg/dL | 8% | 9% |
| Elevated Glucose Levels | |||
| Grade 2 | 6.95–13.88 mmol/L 126–250 mg/dL | 10% | 11% |
| Grade 3 | 13.89–27.75 mmol/L 251–500 mg/dL | 1% | < 1% |
| Grade 4 | > 27.75 mmol/L > 500 mg/dL | < 1% | 0% |
| Pancreatic Lipase | |||
| Grade 2 | > 1.5 to ≤ 3.0 × ULN | 3% | 4% |
| Grade 3 | > 3.0 to ≤ 5.0 × ULN | 2% | < 1% |
| Grade 4 | > 5.0 × ULN | < 1% | 0% |
| Pancreatic Amylase | |||
| Grade 2 | > 1.5 to ≤ 2.0 × ULN | 6% | 7% |
| Grade 3 | > 2.0 to ≤ 5.0 × ULN | 7% | 3% |
| Grade 4 | > 5.0 × ULN | 0% | 0% |
6.3 Serious ADRs
The following serious ADRs of at least moderate intensity (greater than or equal to Grade 2) occurred in the Phase 2b studies and Phase 3 studies with PREZISTA/ritonavir: abdominal pain, acute hepatitis, acute pancreatitis, anorexia, asthenia, diabetes mellitus, diarrhea, fatigue, headache, hepatic enzyme increased, hypercholesterolemia, hyperglycemia, hypertriglyceridemia, immune reconstitution syndrome, low density lipoprotein increased, nausea, pancreatic enzyme increased, rash, Stevens-Johnson Syndrome, and vomiting.
6.4 Patients co-infected with hepatitis B and/or hepatitis C virus
In subjects co-infected with hepatitis B or C virus receiving PREZISTA/ritonavir, the incidence of adverse events and clinical chemistry abnormalities was not higher than in subjects receiving PREZISTA/ritonavir who were not co-infected, except for increased hepatic enzymes [see Warnings and Precautions (5.2)]. The pharmacokinetic exposure in co-infected subjects was comparable to that in subjects without co-infection.
6.5 Clinical Trials Experience: Pediatric Patients
PREZISTA/ritonavir has been studied in combination with other antiretroviral agents in 2 Phase II trials. TMC114-C212, in which 80 antiretroviral treatment-experienced HIV-1-infected pediatric subjects 6 to less than 18 years of age and weighing at least 20 kg were included and TMC114-C228, in which 21 antiretroviral treatment-experienced HIV-1-infected pediatric subjects 3 to less than 6 years of age and weighing at least 10 kg were included [see Use in Specific Populations (8.4) and Clinical Studies (14.4)].
Frequency, type, and severity of ADRs in pediatric subjects were comparable to those observed in adults.
Study TMC114-C212
ADRs to PREZISTA/ritonavir (all grades, greater than or equal to 3%), excluding laboratory abnormalities reported as ADRs, were vomiting (13%), diarrhea (11%), abdominal pain (10%), headache (9%), rash (5%), nausea (4%) and fatigue (3%).
Grade 3 or 4 laboratory abnormalities were ALT increased (Grade 3: 3%; Grade 4: 1%), AST increased (Grade 3: 1%), pancreatic amylase increased (Grade 3: 4%, Grade 4: 1%), pancreatic lipase increased (Grade 3: 1%), total cholesterol increased (Grade 3: 1%), and LDL increased (Grade 3: 3%).
6.6 Postmarketing Experience
The following events have been identified during postmarketing use of PREZISTA. Because these events are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Redistribution of body fat has been reported.
Rarely, rhabdomyolysis (associated with co-administration with HMG-CoA reductase inhibitors and PREZISTA/ritonavir) has been reported.
In addition, toxic epidermal necrolysis and acute generalized exanthematous pustulosis have been reported rarely [see Warnings and Precautions (5.3)].
7 DRUG INTERACTIONS
See also Contraindications (4) and Clinical Pharmacology (12.3).
7.1 Potential for PREZISTA/ritonavir to Affect Other Drugs
PREZISTA co-administered with ritonavir is an inhibitor of CYP3A and CYP2D6. Co-administration of PREZISTA and ritonavir with drugs that are primarily metabolized by CYP3A and CYP2D6 may result in increased plasma concentrations of such drugs, which could increase or prolong their therapeutic effect and adverse events (see Table 9).
7.2 Potential for Other Drugs to Affect Darunavir
Darunavir and ritonavir are metabolized by CYP3A. Drugs that induce CYP3A activity would be expected to increase the clearance of darunavir and ritonavir, resulting in lowered plasma concentrations of darunavir and ritonavir. Co-administration of darunavir and ritonavir and other drugs that inhibit CYP3A may decrease the clearance of darunavir and ritonavir and may result in increased plasma concentrations of darunavir and ritonavir (see Table 9).
7.3 Established and Other Potentially Significant Drug Interactions
Table 9 provides dosing recommendations as a result of drug interactions with PREZISTA/ritonavir. These recommendations are based on either drug interaction studies or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or loss of efficacy.
| Concomitant Drug Class: Drug Name | Effect on Concentration of Darunavir or Concomitant Drug | Clinical Comment |
|---|---|---|
| HIV-1-Antiviral Agents: Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | ||
| didanosine | ↔ darunavir ↔ didanosine | Didanosine should be administered one hour before or two hours after PREZISTA/ritonavir (which are administered with food). |
| HIV-1-Antiviral Agents: HIV-Protease Inhibitors (PIs) | ||
| indinavir (The reference regimen for indinavir was indinavir/ritonavir 800/100 mg twice daily.) | ↑ darunavir ↑ indinavir | The appropriate dose of indinavir in combination with PREZISTA/ritonavir has not been established. |
| lopinavir/ritonavir | ↓ darunavir ↔ lopinavir | Appropriate doses of the combination have not been established. Hence, it is not recommended to co-administer lopinavir/ritonavir and PREZISTA, with or without ritonavir. |
| saquinavir | ↓ darunavir ↔ saquinavir | Appropriate doses of the combination have not been established. Hence, it is not recommended to co-administer saquinavir and PREZISTA, with or without ritonavir. |
| HIV-1-Antiviral Agents: CCR5 co-receptor antagonists | ||
| maraviroc | ↑ maraviroc | Maraviroc concentrations are increased when co-administered with PREZISTA/ritonavir. When used in combination with PREZISTA/ritonavir, the dose of maraviroc should be 150 mg twice daily. |
| Other Agents | ||
| Antiarrhythmics: bepridil, lidocaine (systemic), quinidine, amiodarone, flecainide, propafenone | ↑ antiarrhythmics | Concentrations of these drugs may be increased when co-administered with PREZISTA/ritonavir. Caution is warranted and therapeutic concentration monitoring, if available, is recommended for antiarrhythmics when co-administered with PREZISTA/ritonavir. |
| digoxin | ↑ digoxin | The lowest dose of digoxin should initially be prescribed. The serum digoxin concentrations should be monitored and used for titration of digoxin dose to obtain the desired clinical effect. |
| Anticoagulant: warfarin | ↓ warfarin ↔ darunavir | Warfarin concentrations are decreased when co-administered with PREZISTA/ritonavir. It is recommended that the international normalized ratio (INR) be monitored when warfarin is combined with PREZISTA/ritonavir. |
| Anticonvulsant: carbamazepine | ↔ darunavir ↑ carbamazepine | The dose of either darunavir/ritonavir or carbamazepine does not need to be adjusted when initiating co-administration with darunavir/ritonavir and carbamazepine. Clinical monitoring of carbamazepine concentrations and its dose titration is recommended to achieve the desired clinical response. |
| Anticonvulsant: phenobarbital, phenytoin | ↔ darunavir ↓ phenytoin ↓ phenobarbital | Co-administration of PREZISTA/ritonavir may cause a decrease in the steady-state concentrations of phenytoin and phenobarbital. Phenytoin and phenobarbital levels should be monitored when co-administering with PREZISTA/ritonavir. |
| Antidepressant: trazodone, desipramine | ↑ trazodone ↑ desipramine | Concomitant use of trazodone or desipramine and PREZISTA/ritonavir may increase plasma concentrations of trazodone or desipramine which may lead to adverse events such as nausea, dizziness, hypotension and syncope. If trazodone or desipramine is used with PREZISTA/ritonavir, the combination should be used with caution, and a lower dose of trazodone or desipramine should be considered. |
| Anti-infective: clarithromycin | ↔ darunavir ↑ clarithromycin | No dose adjustment of the combination is required for patients with normal renal function. For patients with renal impairment, the following dose adjustments should be considered:
|
| Antifungals: ketoconazole, itraconazole, voriconazole | ↑ ketoconazole ↑ darunavir ↑ itraconazole (not studied) ↓ voriconazole (not studied) | Ketoconazole and itraconazole are potent inhibitors as well as substrates of CYP3A. Concomitant systemic use of ketoconazole, itraconazole, and darunavir/ritonavir may increase plasma concentration of darunavir. |
| Plasma concentrations of ketoconazole or itraconazole may be increased in the presence of darunavir/ritonavir. When co-administration is required, the daily dose of ketoconazole or itraconazole should not exceed 200 mg. | ||
| Plasma concentrations of voriconazole may be decreased in the presence of darunavir/ritonavir. Voriconazole should not be administered to patients receiving darunavir/ritonavir unless an assessment of the benefit/risk ratio justifies the use of voriconazole. | ||
| Anti-gout: colchicine | ↑ colchicine | Treatment of gout-flares – co-administration of colchicine in patients on PREZISTA/ritonavir:
0.6 mg (1 tablet) × 1 dose, followed by 0.3 mg (half tablet) 1 hour later. Treatment course to be repeated no earlier than 3 days.
Prophylaxis of gout-flares – co-administration of colchicine in patients on PREZISTA/ritonavir:
Treatment of familial Mediterranean fever – co-administration of colchicine in patients on PREZISTA/ritonavir:
|
| Antimycobacterial: rifabutin | ↑ darunavir ↑ rifabutin ↑ 25-O-desacetylrifabutin | Dose reduction of rifabutin by at least 75% of the usual dose (300 mg once daily) is recommended (i.e., a maximum dose of 150 mg every other day). Increased monitoring for adverse events is warranted in patients receiving this combination and further dose reduction of rifabutin may be necessary. |
| The reference regimen for rifabutin was 300 mg once daily | ||
| β-Blockers: metoprolol, timolol | ↑ beta-blockers | Caution is warranted and clinical monitoring of patients is recommended. A dose decrease may be needed for these drugs when co-administered with PREZISTA/ritonavir. |
| Benzodiazepines: parenterally administered midazolam | ↑ midazolam | Concomitant use of parenteral midazolam with PREZISTA/ritonavir may increase plasma concentrations of midazolam. Co-administration should be done in a setting which ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered. Co-administration of oral midazolam with PREZISTA/ritonavir is CONTRAINDICATED. |
| Calcium Channel
Blockers: felodipine, nifedipine, nicardipine | ↑ calcium channel blockers | Plasma concentrations of calcium channel blockers (e.g., felodipine, nifedipine, nicardipine) may increase when PREZISTA/ritonavir are co-administered. Caution is warranted and clinical monitoring of patients is recommended. |
| Corticosteroid: Systemic: dexamethasone | ↓ darunavir | Systemic dexamethasone induces CYP3A and can thereby decrease darunavir plasma concentrations. This may result in loss of therapeutic effect to PREZISTA. |
| Corticosteroid: Inhaled/Nasal: fluticasone | ↑ fluticasone | Concomitant use of inhaled fluticasone and PREZISTA/ritonavir may increase plasma concentrations of fluticasone. Alternatives should be considered, particularly for long-term use. |
| Endothelin receptor antagonists: bosentan | ↑ bosentan |
Co-administration of bosentan in patients on PREZISTA/ritonavir:
Co-administration of PREZISTA/ritonavir in patients on bosentan:
|
| Hepatitis C Virus (HCV) Direct-Acting Agents: NS3-4A protease inhibitors: boceprevir telaprevir | ↓ darunavir ↓ boceprevir ↓ telaprevir |
Concomitant administration of PREZISTA/ritonavir and boceprevir or telaprevir resulted in reduced steady-state exposures to darunavir and boceprevir or telaprevir. It is not recommended to co-administer boceprevir or telaprevir and PREZISTA/ritonavir. |
| HMG-CoA
Reductase Inhibitors: pravastatin, atorvastatin, rosuvastatin | ↑ pravastatin ↑ atorvastatin ↑ rosuvastatin | Titrate atorvastatin, pravastatin or rosuvastatin dose carefully and use the lowest necessary dose while monitoring for safety. Do not exceed atorvastatin 20 mg/day. |
| Immunosuppressants: cyclosporine, tacrolimus, sirolimus | ↑ immunosuppressants | Plasma concentrations of cyclosporine, tacrolimus or sirolimus may be increased when co-administered with PREZISTA/ritonavir. Therapeutic concentration monitoring of the immunosuppressive agent is recommended when co-administered with PREZISTA/ritonavir. |
| Inhaled beta agonist: salmeterol | ↑ salmeterol | Concurrent administration of salmeterol and PREZISTA/ritonavir is not recommended. The combination may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations and sinus tachycardia. |
| Narcotic Analgesic/Treatment of Opioid Dependence: methadone, buprenorphine, buprenorphine/naloxone | ↓ methadone ↔ buprenorphine, naloxone ↑ norbuprenorphine (metabolite) | No adjustment of methadone dosage is required when initiating co-administration of PREZISTA/ritonavir. However, clinical monitoring is recommended as the dose of methadone during maintenance therapy may need to be adjusted in some patients. No dose adjustment for buprenorphine or buprenorphine/naloxone is required with concurrent administration of PREZISTA/ritonavir. Clinical monitoring is recommended if PREZISTA/ritonavir and buprenorphine or buprenorphine/naloxone are coadministered. |
| Neuroleptics: risperidone, thioridazine | ↑ neuroleptics | A dose decrease may be needed for these drugs when co-administered with PREZISTA/ritonavir. |
| Oral Contraceptives/estrogen: ethinyl estradiol, norethindrone | ↓ ethinyl estradiol ↓ norethindrone | Plasma concentrations of ethinyl estradiol are decreased due to induction of its metabolism by ritonavir. Alternative methods of nonhormonal contraception are recommended. |
| PDE-5 inhibitors: sildenafil, vardenafil, tadalafil | ↑ PDE-5 inhibitors (only the use of sildenafil at doses used for treatment of erectile dysfunction has been studied with PREZISTA/ritonavir) |
Co-administration with PREZISTA/ritonavir may result in an increase in PDE-5 inhibitor-associated adverse events, including hypotension, syncope, visual disturbances and priapism. Use of PDE-5 inhibitors for pulmonary arterial hypertension (PAH):
Use of PDE-5 inhibitors for erectile dysfunction:
|
| Selective Serotonin Reuptake Inhibitors (SSRIs): sertraline, paroxetine | ↔ darunavir ↓ sertraline ↓ paroxetine | If sertraline or paroxetine is co-administered with PREZISTA/ritonavir, the recommended approach is a careful dose titration of the SSRI based on a clinical assessment of antidepressant response. In addition, patients on a stable dose of sertraline or paroxetine who start treatment with PREZISTA/ritonavir should be monitored for antidepressant response. |
In addition to the drugs included in Table 9, the interaction between PREZISTA/ritonavir and the following drugs were evaluated in clinical studies and no dose adjustments are needed for either drug [see Clinical Pharmacology (12.3)]: atazanavir, efavirenz, etravirine, nevirapine, omeprazole, ranitidine, rilpivirine, and tenofovir disoproxil fumarate.
Other nucleoside reverse transcriptase inhibitors (NRTIs):
Based on the different elimination pathways of the other NRTIs (zidovudine, zalcitabine, emtricitabine, stavudine, lamivudine and abacavir) that are primarily renally excreted, no drug interactions are expected for these drugs and PREZISTA/ritonavir.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: PREZISTA should be used during pregnancy only if the potential benefit justifies the potential risk.
No adequate and well-controlled studies have been conducted in pregnant women. Reproduction studies conducted with darunavir showed no embryotoxicity or teratogenicity in mice and rats in the presence or absence of ritonavir as well as in rabbits with darunavir alone. In these studies, darunavir exposures (based on AUC) were higher in rats (3-fold), whereas in mice and rabbits, exposures were lower (less than 1-fold) compared to those obtained in humans at the recommended clinical dose of darunavir boosted with ritonavir.
In the rat pre- and postnatal development study, a reduction in pup body weight gain was observed with darunavir alone or in combination with ritonavir during lactation. This was due to exposure of pups to drug substances via the milk. Sexual development, fertility and mating performance of offspring were not affected by maternal treatment with darunavir alone or in combination with ritonavir. The maximal plasma exposures achieved in rats were approximately 50% of those obtained in humans at the recommended clinical dose boosted with ritonavir.
In the juvenile toxicity study where rats were directly dosed with darunavir, deaths occurred from post-natal day 5 through 11 at plasma exposure levels ranging from 0.1 to 1.0 of the human exposure levels. In a 4-week rat toxicology study, when dosing was initiated on post-natal day 23 (the human equivalent of 2 to 3 years of age), no deaths were observed with a plasma exposure (in combination with ritonavir) of 0.1 of the human plasma exposure levels.
8.3 Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV. Although it is not known whether darunavir is secreted in human milk, darunavir is secreted into the milk of lactating rats. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving PREZISTA.
8.4 Pediatric Use
Do not administer PREZISTA/ritonavir in pediatric patients below 3 years of age because of toxicity and mortality observed in juvenile rats dosed with darunavir (from 20 mg/kg to 1000 mg/kg) up to days 23 to 26 of age [see Warnings and Precautions (5.11), Use in Specific Populations (8.1), Clinical Pharmacology (12.3) and Nonclinical Toxicology (13.2)].
Do not administer PREZISTA/ritonavir once daily in pediatric patients.
The safety, pharmacokinetic profile, and virologic and immunologic responses of PREZISTA/ritonavir were evaluated in treatment-experienced HIV-1-infected pediatric subjects 3 to less than 18 years of age and weighing at least 10 kg [see Adverse Reactions (6.6), Clinical Pharmacology (12.3) and Clinical Studies (14.4)]. Frequency, type, and severity of adverse drug reactions in pediatric subjects were comparable to those observed in adults [see Adverse Reactions (6.6)]. Please see Dosage and Administration (2.2) for dosing recommendations for pediatric subjects 3 to less than 18 years of age and weighing at least 10 kg.
8.5 Geriatric Use
Clinical studies of PREZISTA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In general, caution should be exercised in the administration and monitoring of PREZISTA in elderly patients, reflecting the greater frequency of decreased hepatic function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
No dose adjustment of PREZISTA/ritonavir is necessary for patients with either mild or moderate hepatic impairment. No pharmacokinetic or safety data are available regarding the use of PREZISTA/ritonavir in subjects with severe hepatic impairment. Therefore, PREZISTA/ritonavir is not recommended for use in patients with severe hepatic impairment [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Population pharmacokinetic analysis showed that the pharmacokinetics of darunavir were not significantly affected in HIV-infected subjects with moderate renal impairment (CrCL between 30–60 mL/min, n=20). No pharmacokinetic data are available in HIV-1-infected patients with severe renal impairment or end stage renal disease; however, because the renal clearance of darunavir is limited, a decrease in total body clearance is not expected in patients with renal impairment. As darunavir and ritonavir are highly bound to plasma proteins, it is unlikely that they will be significantly removed by hemodialysis or peritoneal dialysis [see Clinical Pharmacology (12.3)].
10 OVERDOSAGE
Human experience of acute overdose with PREZISTA/ritonavir is limited. Single doses up to 3200 mg of the oral solution of darunavir alone and up to 1600 mg of the tablet formulation of darunavir in combination with ritonavir have been administered to healthy volunteers without untoward symptomatic effects.
No specific antidote is available for overdose with PREZISTA. Treatment of overdose with PREZISTA consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. If indicated, elimination of unabsorbed active substance is to be achieved by emesis or gastric lavage. Administration of activated charcoal may also be used to aid in removal of unabsorbed active substance. Since PREZISTA is highly protein bound, dialysis is unlikely to be beneficial in significant removal of the active substance.
11 DESCRIPTION
PREZISTA (darunavir) is an inhibitor of the human immunodeficiency virus (HIV-1) protease.
PREZISTA (darunavir), in the form of darunavir ethanolate, has the following chemical name: [(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-carbamic acid (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester monoethanolate. Its molecular formula is C27H37N3O7S • C2H5OH and its molecular weight is 593.73. Darunavir ethanolate has the following structural formula:
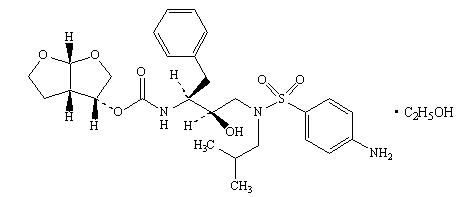
Darunavir ethanolate is a white to off-white powder with a solubility of approximately 0.15 mg/mL in water at 20°C.
PREZISTA 100 mg/mL oral suspension is available as a white to off-white opaque suspension for oral administration.
PREZISTA 75 mg tablets are available as white, caplet-shaped, film-coated tablets for oral administration. PREZISTA 150 mg tablets are available as white, oval-shaped, film-coated tablets for oral administration. PREZISTA 600 mg tablets are available as orange, oval-shaped, film-coated tablets for oral administration. PREZISTA 400 mg tablets are available as light orange, oval-shaped, film-coated tablets for oral administration.
Each mL of the oral suspension contains darunavir ethanolate equivalent to 100 mg darunavir. In addition, each mL contains the inactive ingredients hydroxypropyl cellulose, microcrystalline cellulose, sodium carboxymethylcellulose, methylparaben sodium, citric acid monohydrate, sucralose, masking flavor, strawberry cream flavor, hydrochloric acid (for pH adjustment) and purified water.
Each 75 mg tablet contains darunavir ethanolate equivalent to 75 mg of darunavir. Each 150 mg tablet contains darunavir ethanolate equivalent to 150 mg of darunavir. Each 400 mg tablet contains darunavir ethanolate equivalent to 400 mg of darunavir. Each 600 mg tablet contains darunavir ethanolate equivalent to 600 mg of darunavir. During storage, partial conversion from ethanolate to hydrate may occur; however, this does not affect product quality or performance. Each tablet also contains the inactive ingredients colloidal silicon dioxide, crospovidone, magnesium stearate, and microcrystalline cellulose. The tablet film coating, OPADRY® White, contains polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, and titanium dioxide. The tablet film coating, OPADRY® Orange, contains FD&C Yellow No. 6, polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, and titanium dioxide.
All dosages for PREZISTA are expressed in terms of the free form of darunavir.
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
In an open-label, randomized, placebo- and active-controlled, four-way crossover trial, 40 healthy subjects were administered supratheraputic doses of darunavir/ritonavir 1600/100 mg once daily and 800/100 mg twice daily for seven days.
At the mean maximum darunavir concentration of 6599 ng/mL observed in this study, the mean increase in QTcF was 2.2 ms with a 90% two-sided confidence interval (CI) of -2.0 to 6.3 ms. When evaluating the 2-sided 90% CI on the time-matched mean changes in QTcF versus placebo control, the upper bounds of both darunavir/ritonavir groups never exceeded the 10 ms boundary. In the setting of this trial, darunavir/ritonavir did not appear to prolong the QTc interval.
12.3 Pharmacokinetics
Pharmacokinetics in Adults
General
Darunavir is primarily metabolized by CYP3A. Ritonavir inhibits CYP3A, thereby increasing the plasma concentrations of darunavir. When a single dose of PREZISTA 600 mg was given orally in combination with 100 mg ritonavir twice daily, there was an approximate 14-fold increase in the systemic exposure of darunavir. Therefore, PREZISTA should only be used in combination with 100 mg of ritonavir to achieve sufficient exposures of darunavir.
The pharmacokinetics of darunavir, co-administered with low dose ritonavir (100 mg), has been evaluated in healthy adult volunteers and in HIV-1-infected subjects. Table 10 displays the population pharmacokinetic estimates of darunavir after oral administration of PREZISTA/ritonavir 600/100 mg twice daily [based on sparse sampling in 285 patients in study TMC114-C214, 278 patients in Study TMC114-C229 and 119 patients (integrated data) from Studies TMC114-C202 and TMC114-C213] and PREZISTA/ritonavir 800/100 mg once daily [based on sparse sampling in 335 patients in Study TMC114-C211 and 280 patients in Study TMC114-C229] to HIV-1-infected patients.
| Parameter | Study TMC114-C211 PREZISTA/ ritonavir 800/100 mg once daily N = 335 | Study TMC114-C229 PREZISTA/ ritonavir 800/100 mg once daily N = 280 | Study TMC114-C214 PREZISTA/ ritonavir 600/100 mg twice daily N = 285 | Study TMC114-C229 PREZISTA/ ritonavir 600/100 mg twice daily N = 278 | Studies TMC114-C213 and TMC114-C202 (integrated data) PREZISTA/ ritonavir 600/100 mg twice daily N =119 |
|---|---|---|---|---|---|
| N = number of subjects with data | |||||
|
|||||
| AUC24h (ng∙h/mL)* | |||||
| Mean ± Standard Deviation | 93026 ± 27050 | 93334 ± 28626 | 116796 ± 33594 | 114302 ± 32681 | 124698 ± 32286 |
| Median (Range) | 87854 (45000–219240) | 87788 (45456–236920) | 111632 (64874–355360) | 109401 (48934–323820) | 123336 (67714–212980) |
| C0h (ng/mL) | |||||
| Mean ± Standard Deviation | 2282 ± 1168 | 2160 ± 1201 | 3490 ± 1401 | 3386 ± 1372 | 3578 ± 1151 |
| Median (Range) | 2041 (368–7242) | 1896 (184–7881) | 3307 (1517–13198) | 3197 (250–11865) | 3539 (1255–7368) |
Absorption and Bioavailability
Darunavir, co-administered with 100 mg ritonavir twice daily, was absorbed following oral administration with a Tmax of approximately 2.5–4 hours. The absolute oral bioavailability of a single 600 mg dose of darunavir alone and after co-administration with 100 mg ritonavir twice daily was 37% and 82%, respectively. In vivo data suggest that darunavir/ritonavir is an inhibitor of the p-glycoprotein (p-gp) transporters.
Effects of Food on Oral Absorption
When PREZISTA tablets were administered with food, the Cmax and AUC of darunavir, co-administered with ritonavir, is approximately 40% higher relative to the fasting state. Therefore, PREZISTA tablets, co-administered with ritonavir, should always be taken with food. Within the range of meals studied, darunavir exposure is similar. The total caloric content of the various meals evaluated ranged from 240 Kcal (12 gms fat) to 928 Kcal (56 gms fat).
Distribution
Darunavir is approximately 95% bound to plasma proteins. Darunavir binds primarily to plasma alpha 1-acid glycoprotein (AAG).
Metabolism
In vitro experiments with human liver microsomes (HLMs) indicate that darunavir primarily undergoes oxidative metabolism. Darunavir is extensively metabolized by CYP enzymes, primarily by CYP3A. A mass balance study in healthy volunteers showed that after a single dose administration of 400 mg 14C-darunavir, co-administered with 100 mg ritonavir, the majority of the radioactivity in the plasma was due to darunavir. At least 3 oxidative metabolites of darunavir have been identified in humans; all showed activity that was at least 90% less than the activity of darunavir against wild-type HIV-1.
Elimination
A mass balance study in healthy volunteers showed that after single dose administration of 400 mg 14C-darunavir, co-administered with 100 mg ritonavir, approximately 79.5% and 13.9% of the administered dose of 14C-darunavir was recovered in the feces and urine, respectively. Unchanged darunavir accounted for approximately 41.2% and 7.7% of the administered dose in feces and urine, respectively. The terminal elimination half-life of darunavir was approximately 15 hours when co-administered with ritonavir. After intravenous administration, the clearance of darunavir, administered alone and co-administered with 100 mg twice daily ritonavir, was 32.8 L/h and 5.9 L/h, respectively.
Special Populations
Hepatic Impairment
Darunavir is primarily metabolized by the liver. The steady-state pharmacokinetic parameters of darunavir were similar after multiple dose co-administration of PREZISTA/ritonavir 600/100 mg twice daily to subjects with normal hepatic function (n=16), mild hepatic impairment (Child-Pugh Class A, n=8), and moderate hepatic impairment (Child-Pugh Class B, n=8). The effect of severe hepatic impairment on the pharmacokinetics of darunavir has not been evaluated [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)].
Hepatitis B or Hepatitis C Virus Co-infection
The 48-week analysis of the data from Studies TMC114-C211 and TMC114-C214 in HIV-1-infected subjects indicated that hepatitis B and/or hepatitis C virus co-infection status had no apparent effect on the exposure of darunavir.
Renal Impairment
Results from a mass balance study with 14C-darunavir/ritonavir showed that approximately 7.7% of the administered dose of darunavir is excreted in the urine as unchanged drug. As darunavir and ritonavir are highly bound to plasma proteins, it is unlikely that they will be significantly removed by hemodialysis or peritoneal dialysis. Population pharmacokinetic analysis showed that the pharmacokinetics of darunavir were not significantly affected in HIV-1-infected subjects with moderate renal impairment (CrCL between 30–60 mL/min, n=20). There are no pharmacokinetic data available in HIV-1-infected patients with severe renal impairment or end stage renal disease [see Use in Specific Populations (8.7)].
Gender
Population pharmacokinetic analysis showed higher mean darunavir exposure in HIV-1-infected females compared to males. This difference is not clinically relevant.
Race
Population pharmacokinetic analysis of darunavir in HIV-1-infected subjects indicated that race had no apparent effect on the exposure to darunavir.
Geriatric Patients
Population pharmacokinetic analysis in HIV-1-infected subjects showed that darunavir pharmacokinetics are not considerably different in the age range (18 to 75 years) evaluated in HIV-1-infected subjects (n = 12, age greater than or equal to 65) [see Use in Specific Populations (8.5)].
Pediatric Patients
The pharmacokinetics of darunavir in combination with ritonavir in 92 antiretroviral treatment-experienced HIV-1-infected pediatric subjects 3 to less than 18 years of age and weighing at least 10 kg showed that the administered weight-based dosages resulted in darunavir exposure that was comparable to the exposures achieved in treatment-experienced adults receiving PREZISTA/ritonavir 600/100 mg twice daily [see Dosage and Administration (2.2)].
| Parameter | Study TMC114-C212 PREZISTA/ ritonavir twice daily N = 74 | Study TMC114-C228 PREZISTA/ ritonavir twice daily |
|
|---|---|---|---|
| 10 to less than 15 kg
N = 10 | 15 to less than 20 kg
N = 12 |
||
| N = number of subjects with data. | |||
| AUC24h (ng∙h/mL) | |||
| Mean ± Standard Deviation | 126377 (34356) | 137399 ± 51067 | 158773 ± 61932 |
| Median (Range) | 127340 (67054–230720) | 123229 (92098–262720) | 138578 (104974–317420) |
| C0h (ng/mL) | |||
| Mean ± Standard Deviation | 3948 (1363) | 4429 ± 2064 | 4858 ± 2521 |
| Median (Range) | 3888 (1836–7821) | 4010 (2576–9488) | 4469 (2733–11300) |
Drug Interactions
[See also Contraindications (4), Warnings and Precautions (5.5), and Drug Interactions (7).]
Darunavir co-administered with ritonavir is an inhibitor of CYP3A and CYP2D6. Co-administration of darunavir and ritonavir with drugs primarily metabolized by CYP3A and CYP2D6 may result in increased plasma concentrations of such drugs, which could increase or prolong their therapeutic effect and adverse events.
Darunavir and ritonavir are metabolized by CYP3A. Drugs that induce CYP3A activity would be expected to increase the clearance of darunavir and ritonavir, resulting in lowered plasma concentrations of darunavir and ritonavir. Co-administration of darunavir and ritonavir and other drugs that inhibit CYP3A may decrease the clearance of darunavir and ritonavir and may result in increased plasma concentrations of darunavir and ritonavir.
Drug interaction studies were performed with darunavir and other drugs likely to be co-administered and some drugs commonly used as probes for pharmacokinetic interactions. The effects of co-administration of darunavir on the AUC, Cmax, and Cmin values are summarized in Table 12 (effect of other drugs on darunavir) and Table 13 (effect of darunavir on other drugs). For information regarding clinical recommendations, see Drug Interactions (7).
Several interaction studies have been performed with a dose other than the recommended dose of the co-administered drug or darunavir; however, the results are applicable to the recommended dose of the co-administered drug and/or darunavir.
| Co-Administered Drug | Dose/Schedule | N | PK | LS Mean Ratio (90% CI) of Darunavir Pharmacokinetic Parameters With/Without Co-administered Drug No Effect =1.00 |
|||
|---|---|---|---|---|---|---|---|
| Co-Administered Drug | Darunavir/ ritonavir | Cmax | AUC | Cmin | |||
| N = number of subjects with data | |||||||
|
|||||||
| Co-Administration With Other HIV Protease Inhibitors | |||||||
| Atazanavir | 300 mg q.d.* | 400/100 mg b.i.d. † | 13 | ↔ | 1.02 (0.96–1.09) | 1.03 (0.94–1.12) | 1.01 (0.88–1.16) |
| Indinavir | 800 mg b.i.d. | 400/100 mg b.i.d. | 9 | ↑ | 1.11 (0.98–1.26) | 1.24 (1.09–1.42) | 1.44 (1.13–1.82) |
| Lopinavir/ Ritonavir | 400/100 mg b.i.d. | 1200/100 mg b.i.d.‡ | 14 | ↓ | 0.79 (0.67–0.92) | 0.62 (0.53–0.73) | 0.49 (0.39–0.63) |
| 533/133.3 mg b.i.d. | 1200 mg b.i.d.‡ | 15 | ↓ | 0.79 (0.64–0.97) | 0.59 (0.50–0.70) | 0.45 (0.38–0.52) |
|
| Saquinavir hard gel capsule | 1000 mg b.i.d. | 400/100 mg b.i.d. | 14 | ↓ | 0.83 (0.75–0.92) | 0.74 (0.63–0.86) | 0.58 (0.47–0.72) |
| Co-Administration With Other HIV Antiretrovirals | |||||||
| Didanosine | 400 mg q.d. | 600/100 mg b.i.d. | 17 | ↔ | 0.93 (0.86–1.00) | 1.01 (0.95–1.07) | 1.07 (0.95–1.21) |
| Efavirenz | 600 mg q.d. | 300/100 mg b.i.d. | 12 | ↓ | 0.85 (0.72–1.00) | 0.87 (0.75–1.01) | 0.69 (0.54–0.87) |
| Etravirine | 200 mg b.i.d. | 600/100 mg b.i.d. | 15 | ↔ | 1.11 (1.01–1.22) | 1.15 (1.05–1.26) | 1.02 (0.90–1.17) |
| Nevirapine | 200 mg b.i.d. | 400/100 mg b.i.d. | 8 | ↑ | 1.40 §
(1.14–1.73) | 1.24 §
(0.97–1.57) | 1.02 §
(0.79–1.32) |
| Rilpivirine | 150 mg q.d. | 800/100 mg q.d. | 15 | ↔ | 0.90 (0.81–1.00) | 0.89 (0.81–0.99) | 0.89 (0.68–1.16) |
| Tenofovir Disoproxil Fumarate | 300 mg q.d. | 300/100 mg b.i.d. | 12 | ↑ | 1.16 (0.94–1.42) | 1.21 (0.95–1.54) | 1.24 (0.90–1.69) |
| Co-Administration With HCV NS3-4A Protease Inhibitors | |||||||
| Boceprevir¶ | 800 mg three times daily | 600/100 mg b.i.d. | 11 | ↓ | 0.64 (0.58–0.71) | 0.56 (0.51–0.61) | 0.41 (0.38–0.45) |
| Telaprevir | 750 mg every 8 hours 1125 mg every 12 hours | 600/100 mg b.i.d. 600/100 mg b.i.d. | 11#
15 | ↓ ↓ | 0.60 (0.56–0.64) 0.53 (0.47–0.59) | 0.60 (0.57–0.63) 0.49 (0.43–0.55) | 0.58 (0.52–0.64) 0.42 (0.35–0.51) |
| Co-Administration With Other Drugs | |||||||
| Carbamazepine | 200 mg b.i.d. | 600/100 mg b.i.d. | 16 | ↔ | 1.04 (0.93–1.16) | 0.99 (0.90–1.08) | 0.85 (0.73–1.00) |
| Clarithromycin | 500 mg b.i.d. | 400/100 mg b.i.d. | 17 | ↔ | 0.83 (0.72–0.96) | 0.87 (0.75–1.01) | 1.01 (0.81–1.26) |
| Ketoconazole | 200 mg b.i.d. | 400/100 mg b.i.d. | 14 | ↑ | 1.21 (1.04–1.40) | 1.42 (1.23–1.65) | 1.73 (1.39–2.14) |
| Omeprazole | 20 mg q.d. | 400/100 mg b.i.d. | 16 | ↔ | 1.02 (0.95–1.09) | 1.04 (0.96–1.13) | 1.08 (0.93–1.25) |
| Paroxetine | 20 mg q.d. | 400/100 mg b.i.d. | 16 | ↔ | 0.97 (0.92–1.02) | 1.02 (0.95–1.10) | 1.07 (0.96–1.19) |
| Ranitidine | 150 mg b.i.d. | 400/100 mg b.i.d. | 16 | ↔ | 0.96 (0.89–1.05) | 0.95 (0.90–1.01) | 0.94 (0.90–0.99) |
| Rifabutin | 150 mg q.o.d. Þ | 600/100 mg b.i.d. | 11 | ↑ | 1.42 (1.21–1.67) | 1.57 (1.28–1.93) | 1.75 (1.28–2.37) |
| Sertraline | 50 mg q.d. | 400/100 mg b.i.d. | 13 | ↔ | 1.01 (0.89–1.14) | 0.98 (0.84–1.14) | 0.94 (0.76–1.16) |
| Co-Administered Drug | Dose/Schedule | N | PK | LS Mean Ratio (90% CI) of Co-Administered Drug Pharmacokinetic Parameters With/Without Darunavir No effect =1.00 |
|||
|---|---|---|---|---|---|---|---|
| Co-Administered Drug | Darunavir/ ritonavir | Cmax | AUC | Cmin | |||
| N = number of subjects with data;- = no information available | |||||||
| A cocktail study was conducted in 12 healthy volunteers to evaluate the effect of steady state pharmacokinetics of darunavir/ritonavir on the activity of CYP2D6 (using dextromethorphan as probe substrate), CYP2C9 (using warfarin as probe substrate), and CYP2C19 (using omeprazole as probe substrate). The pharmacokinetic results are shown in Table 13. | |||||||
|
|||||||
| Co-Administration With Other HIV Protease Inhibitors | |||||||
| Atazanavir | 300 mg q.d.* /100 mg ritonavir q.d. when administered alone | 400/100 mg b.i.d. † | 13 | ↔ | 0.89 (0.78–1.01) | 1.08 (0.94–1.24) | 1.52 (0.99–2.34) |
| 300 mg q.d. when administered with darunavir/ritonavir | |||||||
| Indinavir | 800 mg b.i.d. /100 mg ritonavir b.i.d. when administered alone | 400/100 mg b.i.d. | 9 | ↑ | 1.08 (0.95–1.22) | 1.23 (1.06–1.42) | 2.25 (1.63–3.10) |
| 800 mg b.i.d. when administered with darunavir/ ritonavir | |||||||
| Lopinavir/Ritonavir | 400/100 mg b.i.d.‡ | 1200/100 mg b.i.d. | 14 | ↔ | 0.98 (0.78–1.22) | 1.09 (0.86–1.37) | 1.23 (0.90–1.69) |
| 533/133.3 mg b.i.d.‡ | 1200 mg b.i.d. | 15 | ↔ | 1.11 (0.96–1.30) | 1.09 (0.96–1.24) | 1.13 (0.90–1.42) |
|
| Saquinavir hard gel capsule | 1000 mg b.i.d. /100 mg ritonavir b.i.d. when administered alone | 400/100 mg b.i.d. | 12 | ↔ | 0.94 (0.78–1.13) | 0.94 (0.76–1.17) | 0.82 (0.52–1.30) |
| 1000 mg b.i.d. when administered with darunavir/ritonavir | |||||||
| Co-Administration With Other HIV Antiretrovirals | |||||||
| Didanosine | 400 mg q.d. | 600/100 mg b.i.d. | 17 | ↔ | 0.84 (0.59–1.20) | 0.91 (0.75–1.10) | - |
| Efavirenz | 600 mg q.d. | 300/100 mg b.i.d. | 12 | ↑ | 1.15 (0.97–1.35) | 1.21 (1.08–1.36) | 1.17 (1.01–1.36) |
| Etravirine | 100 mg b.i.d. | 600/100 mg b.i.d. | 14 | ↓ | 0.68 (0.57–0.82) | 0.63 (0.54–0.73) | 0.51 (0.44–0.61) |
| Nevirapine | 200 mg b.i.d. | 400/100 mg b.i.d. | 8 | ↑ | 1.18 (1.02–1.37) | 1.27 (1.12–1.44) | 1.47 (1.20–1.82) |
| Rilpivirine | 150 mg q.d. | 800/100 mg q.d. | 14 | ↑ | 1.79 (1.56–2.06) | 2.30 (1.98–2.67) | 2.78 (2.39–3.24) |
| Tenofovir Disoproxil Fumarate | 300 mg q.d. | 300/100 mg b.i.d. | 12 | ↑ | 1.24 (1.08–1.42) | 1.22 (1.10–1.35) | 1.37 (1.19–1.57) |
| Maraviroc | 150 mg b.i.d. | 600/100 mg b.i.d. | 12 | ↑ | 2.29 (1.46–3.59) | 4.05 (2.94–5.59) | 8.00 (6.35–10.1) |
| Maraviroc | 150 mg b.i.d. | 600/100 mg b.i.d. with 200 mg b.i.d. etravirine | 10 | ↑ | 1.77 (1.20–2.60) | 3.10 (2.57–3.74) | 5.27 (4.51–6.15) |
| Co-Administration With HCV NS3-4A Protease Inhibitors | |||||||
| Boceprevir | 800 mg three times daily | 600/100 mg b.i.d. | 12§ | ↓ | 0.75 (0.67–0.85) | 0.68 (0.65–0.72) | 0.65 (0.56–0.76) |
| Telaprevir | 750 mg every 8 hours | 600/100 mg b.i.d. | 11¶ | ↓ | 0.64 (0.61–0.67) | 0.65 (0.61–0.69) | 0.68 (0.63–0.74) |
| Co-Administration With Other Drugs | |||||||
| Atorvastatin | 40 mg q.d. when administered alone | 300/100 mg b.i.d. | 15 | ↑ | 0.56 (0.48–0.67) | 0.85 (0.76–0.97) | 1.81 (1.37–2.40) |
| 10 mg q.d. when administered with darunavir/ritonavir | |||||||
| Buprenorphine/ Naloxone | 8/2 mg to 16/4 mg q.d. | 600/100 mg b.i.d. | 17 | ↔ | 0.92 #
(0.79–1.08) | 0.89 #
(0.78–1.02) | 0.98 #
(0.82–1.16) |
| Norbuprenorphine | 17 | ↑ | 1.36 (1.06–1.74) | 1.46 (1.15–1.85) | 1.71 (1.29–2.27) |
||
| Carbamazepine | 200 mg b.i.d. | 600/100 mg b.i.d. | 16 | ↑ | 1.43 (1.34–1.53) | 1.45 (1.35–1.57) | 1.54 (1.41–1.68) |
| Carbamazepine epoxide | 16 | ↓ | 0.46 (0.43–0.49) | 0.46 (0.44–0.49) | 0.48 (0.45–0.51) |
||
| Clarithromycin | 500 mg b.i.d. | 400/100 mg b.i.d. | 17 | ↑ | 1.26 (1.03–1.54) | 1.57 (1.35–1.84) | 2.74 (2.30–3.26) |
| Dextromethorphan | 30 mg | 600/100 mg b.i.d. | 12 | ↑ | 2.27 (1.59–3.26) | 2.70 (1.80–4.05) | - |
| Dextrorphan | ↓ | 0.87 (0.77–0.98) | 0.96 (0.90–1.03) | - | |||
| Digoxin | 0.4 mg | 600/100 mg b.i.d. | 8 | ↑ | 1.15 (0.89–1.48) | 1.36 (0.81–2.27) | - |
| Ethinyl estradiol (EE) | Ortho-Novum 1/35 (35 µg EE / 1 mg NE) | 600/100 mg b.i.d. | 11 | ↓ | 0.68 (0.61–0.74) | 0.56 (0.50–0.63) | 0.38 (0.27–0.54) |
| Norethindrone (NE) | 11 | ↓ | 0.90 (0.83–0.97) | 0.86 (0.75–0.98) | 0.70 (0.51–0.97) |
||
| Ketoconazole | 200 mg b.i.d. | 400/100 mg b.i.d. | 15 | ↑ | 2.11 (1.81–2.44) | 3.12 (2.65–3.68) | 9.68 (6.44–14.55) |
| R-Methadone | 55–150 mg q.d. | 600/100 mg b.i.d. | 16 | ↓ | 0.76 (0.71–0.81) | 0.84 (0.78–0.91) | 0.85 (0.77–0.94) |
| Omeprazole | 40 mg single dose | 600/100 mg b.i.d. | 12 | ↓ | 0.66 (0.48–0.90) | 0.58 (0.50–0.66) | - |
| 5-hydroxy omeprazole | ↓ | 0.93 (0.71–1.21) | 0.84 (0.77–0.92) | - | |||
| Paroxetine | 20 mg q.d. | 400/100 mg b.i.d. | 16 | ↓ | 0.64 (0.59–0.71) | 0.61 (0.56–0.66) | 0.63 (0.55–0.73) |
| Pravastatin | 40 mg single dose | 600/100 mg b.i.d. | 14 | ↑ | 1.63 (0.95–2.82) | 1.81 (1.23–2.66) | - |
| Rifabutin | 150 mg q.o.d. Þ when administered with PREZISTA/ritonavir | 600/100 mg b.i.d. ß | 11 | ↑ | 0.72 (0.55–0.93) | 0.93 (0.80–1.09) | 1.64 (1.48–1.81) |
| 25-O-desacetyl-rifabutin | 300 mg q.d. when administered alone | 11 | ↑ | 4.77 (4.04–5.63) | 9.81 (8.09–11.9) | 27.1 (22.2–33.2) |
|
| Sertraline | 50 mg q.d. | 400/100 mg b.i.d. | 13 | ↓ | 0.56 (0.49–0.63) | 0.51 (0.46–0.58) | 0.51 (0.45–0.57) |
| Sildenafil | 100 mg (single dose) administered alone | 400/100 mg b.i.d. | 16 | ↑ | 0.62 (0.55–0.70) | 0.97 (0.86–1.09) | - |
| 25 mg (single dose) when administered with darunavir/ritonavir | |||||||
| S-warfarin | 10 mg single dose | 600/100 mg b.i.d. | 12 | ↓ | 0.92 (0.86–0.97) | 0.79 (0.73–0.85) | - |
| 7-OH-S-warfarin | 12 | ↑ | 1.42 (1.24–1.63) | 1.23 (0.97–1.57) | - | ||
12.4 Microbiology
Mechanism of Action
Darunavir is an inhibitor of the HIV-1 protease. It selectively inhibits the cleavage of HIV-1 encoded Gag-Pol polyproteins in infected cells, thereby preventing the formation of mature virus particles.
Antiviral Activity
Darunavir exhibits activity against laboratory strains and clinical isolates of HIV-1 and laboratory strains of HIV-2 in acutely infected T-cell lines, human peripheral blood mononuclear cells and human monocytes/macrophages with median EC50 values ranging from 1.2 to 8.5 nM (0.7 to 5.0 ng/mL). Darunavir demonstrates antiviral activity in cell culture against a broad panel of HIV-1 group M (A, B, C, D, E, F, G), and group O primary isolates with EC50 values ranging from less than 0.1 to 4.3 nM. The EC50 value of darunavir increases by a median factor of 5.4 in the presence of human serum. Darunavir did not show antagonism when studied in combination with the PIs amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, or tipranavir, the N(t)RTIs abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zalcitabine, or zidovudine, the NNRTIs delavirdine, efavirenz, etravirine, or nevirapine, and the fusion inhibitor enfuvirtide.
Resistance
Cell Culture: HIV-1 isolates with a decreased susceptibility to darunavir have been selected in cell culture and obtained from subjects treated with darunavir/ritonavir. Darunavir-resistant virus derived in cell culture from wild-type HIV-1 had 21- to 88-fold decreased susceptibility to darunavir and developed 2 to 4 of the following amino acid substitutions S37D, R41E/T, K55Q, H69Q, K70E, T74S, V77I, or I85V in the protease. Selection in cell culture of darunavir resistant HIV-1 from nine HIV-1 strains harboring multiple PI resistance-associated mutations resulted in the overall emergence of 22 mutations in the protease gene, coding for amino acid substitutions L10F, V11I, I13V, I15V, G16E, L23I, V32I, L33F, S37N, M46I, I47V, I50V, F53L, L63P, A71V, G73S, L76V, V82I, I84V, T91A/S, and Q92R, of which L10F, V32I, L33F, S37N, M46I, I47V, I50V, L63P, A71V, and I84V were the most prevalent. These darunavir-resistant viruses had at least eight protease substitutions and exhibited 50- to 641-fold decreases in darunavir susceptibility with final EC50 values ranging from 125 nM to 3461 nM.
Clinical studies of PREZISTA/ritonavir in treatment-experienced subjects: In a pooled analysis of the 600/100 mg PREZISTA/ritonavir twice daily arms of Studies TMC114-C213, TMC114-C202, TMC114-C215, and the control arms of etravirine studies TMC125-C206 and TMC125-C216, the amino acid substitutions V32I and I54L or M developed most frequently on PREZISTA/ritonavir in 41% and 25%, respectively, of the treatment-experienced subjects who experienced virologic failure, either by rebound or by never being suppressed (less than 50 copies/mL). Other substitutions that developed frequently in PREZISTA/ritonavir virologic failure isolates occurred at amino acid positions V11I, I15V, L33F, I47V, I50V, and L89V. These amino acid substitutions were associated with decreased susceptibility to darunavir; 90% of the virologic failure isolates had a greater than 7-fold decrease in susceptibility to darunavir at failure. The median darunavir phenotype (fold change from reference) of the virologic failure isolates was 4.3-fold at baseline and 85-fold at failure. Amino acid substitutions were also observed in the protease cleavage sites in the Gag polyprotein of some PREZISTA/ritonavir virologic failure isolates. In Study TMC114-C212 of treatment-experienced pediatric subjects, the amino acid substitutions V32I, I54L and L89M developed most frequently in virologic failures on PREZISTA/ritonavir.
In the 96-week as-treated analysis of the Phase 3 Study TMC114-C214, the percent of virologic failures (never suppressed, rebounders and discontinued before achieving suppression) was 21% (62/298) in the group of subjects receiving PREZISTA/ritonavir 600/100 mg twice daily compared to 32% (96/297) of subjects receiving lopinavir/ritonavir 400/100 mg twice daily. Examination of subjects who failed on PREZISTA/ritonavir 600/100 mg twice daily and had post-baseline genotypes and phenotypes showed that 7 subjects (7/43; 16%) developed PI substitutions on darunavir/ritonavir treatment resulting in decreased susceptibility to darunavir. Six of the 7 had baseline PI resistance-associated substitutions and baseline darunavir phenotypes greater than 7. The most common emerging PI substitutions in these virologic failures were V32I, L33F, M46I or L, I47V, I54L, T74P and L76V. These amino acid substitutions were associated with 59- to 839-fold decreased susceptibility to darunavir at failure. Examination of individual subjects who failed in the comparator arm on lopinavir/ritonavir and had post-baseline genotypes and phenotypes showed that 31 subjects (31/75; 41%) developed substitutions on lopinavir treatment resulting in decreased susceptibility to lopinavir (greater than 10-fold) and the most common substitutions emerging on treatment were L10I or F, M46I or L, I47V or A, I54V and L76V. Of the 31 lopinavir/ritonavir virologic failure subjects, 14 had reduced susceptibility (greater than 10-fold) to lopinavir at baseline.
In the 48-week analysis of the Phase 3 Study TMC114-C229, the number of virologic failures (including those who discontinued before suppression after Week 4) was 26% (75/294) in the group of subjects receiving PREZISTA/ritonavir 800/100 mg once daily compared to 19% (56/296) of subjects receiving PREZISTA/ritonavir 600/100 mg twice daily. Examination of isolates from subjects who failed on PREZISTA/ritonavir 800/100 mg once daily and had post-baseline genotypes showed that 8 subjects (8/60; 13%) had isolates that developed IAS-USA defined PI resistance-associated substitutions compared to 5 subjects (5/39; 13%) on PREZISTA/ritonavir 600/100 mg twice daily. Isolates from 2 subjects developed PI resistance associated substitutions associated with decreased susceptibility to darunavir; 1 subject isolate in the PREZISTA/ritonavir 800/100 mg once daily arm, developed substitutions V32I, M46I, L76V and I84V associated with a 24-fold decreased susceptibility to darunavir, and 1 subject isolate in the PREZISTA/ritonavir 600/100 mg twice daily arm developed substitutions L33F and I50V associated with a 40-fold decreased susceptibility to darunavir. In the PREZISTA/ritonavir 800/100 mg once daily and PREZISTA/ritonavir 600/100 mg twice daily groups, isolates from 7 (7/60, 12%) and 4 (4/42, 10%) virologic failures, respectively, developed decreased susceptibility to an NRTI included in the treatment regimen.
Clinical studies of PREZISTA/ritonavir in treatment-naive subjects: In the 192-week as-treated analysis censoring those who discontinued before Week 4 of the Phase 3 Study TMC114-C211, the percentage of virologic failures (never suppressed, rebounders and discontinued before achieving suppression) was 22% (64/288) in the group of subjects receiving PREZISTA/ritonavir 800/100 mg once daily compared to 29% (76/263) of subjects receiving lopinavir/ritonavir 800/200 mg per day. In the PREZISTA/ritonavir arm, emergent PI resistance-associated substitutions were identified in 11 of the virologic failures with post-baseline genotypic data (n=43). However, none of the darunavir virologic failures had a decrease in darunavir susceptibility (greater than 7-fold change) at failure. In the comparator lopinavir/ritonavir arm, emergent PI resistance-associated substitutions were identified in 17 of the virologic failures with post-baseline genotypic data (n=53), but none of the lopinavir/ritonavir virologic failures had decreased susceptibility to lopinavir (greater than 10-fold change) at failure. The reverse transcriptase M184V substitution and/or resistance to emtricitabine, which was included in the fixed background regimen, was identified in 4 virologic failures from the PREZISTA/ritonavir arm and 7 virologic failures in the lopinavir/ritonavir arm.
Cross-resistance
Cross-resistance among PIs has been observed. Darunavir has a less than 10-fold decreased susceptibility in cell culture against 90% of 3309 clinical isolates resistant to amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir and/or tipranavir showing that viruses resistant to these PIs remain susceptible to darunavir.
Darunavir-resistant viruses were not susceptible to amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir or saquinavir in cell culture. However, six of nine darunavir-resistant viruses selected in cell culture from PI-resistant viruses showed a fold change in EC50 values less than 3 for tipranavir, indicative of limited cross-resistance between darunavir and tipranavir. In Studies TMC114-C213, TMC114-C202, and TMC114-C215, 34% (64/187) of subjects in the darunavir/ritonavir arm whose baseline isolates had decreased susceptibility to tipranavir (tipranavir fold change greater than 3) achieved less than 50 copies/mL serum HIV-1 RNA levels at Week 96. Of the viruses isolated from subjects experiencing virologic failure on PREZISTA/ritonavir 600/100 mg twice daily (greater than 7 fold change), 41% were still susceptible to tipranavir and 10% were susceptible to saquinavir while less than 2% were susceptible to the other protease inhibitors (amprenavir, atazanavir, indinavir, lopinavir or nelfinavir).
In Study TMC114-C214, the 7 darunavir/ritonavir virologic failures with reduced susceptibility to darunavir at failure were also resistant to the approved PIs (fos)amprenavir, atazanavir, lopinavir, indinavir, and nelfinavir at failure. Six of these 7 were resistant to saquinavir and 5 were resistant to tipranavir. Four of these virologic failures were already PI-resistant at baseline.
Cross-resistance between darunavir and nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, fusion inhibitors, CCR5 co-receptor antagonists, or integrase inhibitors is unlikely because the viral targets are different.
Baseline Genotype/Phenotype and Virologic Outcome Analyses
Genotypic and/or phenotypic analysis of baseline virus may aid in determining darunavir susceptibility before initiation of PREZISTA/ritonavir 600/100 mg twice daily therapy. The effect of baseline genotype and phenotype on virologic response at 96 weeks was analyzed in as-treated analyses using pooled data from the Phase 2b studies (Studies TMC114-C213, TMC114-C202, and TMC114-C215) (n=439). The findings were confirmed with additional genotypic and phenotypic data from the control arms of etravirine Studies TMC125-C206 and TMC125-C216 at Week 24 (n=591).
Diminished virologic responses were observed in subjects with 5 or more baseline IAS-defined primary protease inhibitor resistance-associated substitutions (D30N, V32I, L33F, M46I/L, I47A/V, G48V, I50L/V, I54L/M, L76V, V82A/F/L/S/T, I84V, N88S, L90M) (see Table 14).
| Studies TMC114-C213, TMC114-C202, TMC114-C215 < 50 copies/mL at Week 96 N=439 |
|||
|---|---|---|---|
| # IAS-Defined Primary PI Substitutions | Overall | De Novo ENF | Re-Used/ No ENF |
| IAS Primary PI Substitutions (2008): D30N, V32I, L33F, M46I/L, I47A/V, G48V, I50L/V, I54L/M, L76V, V82A/F/L/S/T, I84V, N88S, L90M | |||
| All | 44% (192/439) | 54% (61/112) | 40% (131/327) |
| 0 – 4 | 50% (162/322) | 58% (49/85) | 48% (113/237) |
| 5 | 22% (16/74) | 47% (9/19) | 13% (7/55) |
| ≥ 6 | 9% (3/32) | 17% (1/6) | 8% (2/26) |
The presence at baseline of two or more of the substitutions V11I, V32I, L33F, I47V, I50V, I54L or M, T74P, L76V, I84V or L89V was associated with a decreased virologic response to PREZISTA/ritonavir. In subjects not taking enfuvirtide de novo, the proportion of subjects achieving viral load less than 50 plasma HIV-1 RNA copies/mL at 96 weeks was 59%, 29%, and 12% when the baseline genotype had 0–1, 2 and greater than or equal to 3 of these substitutions, respectively.
Baseline darunavir phenotype (shift in susceptibility relative to reference) was shown to be a predictive factor of virologic outcome. Response rates assessed by baseline darunavir phenotype are shown in Table 15. These baseline phenotype groups are based on the select patient populations in the Studies TMC114-C213, TMC114-C202, and TMC114-C215, and are not meant to represent definitive clinical susceptibility breakpoints for PREZISTA/ritonavir. The data are provided to give clinicians information on the likelihood of virologic success based on pre-treatment susceptibility to darunavir.
| Proportion of Subjects with < 50 copies/mL at Week 96 N=417 |
|||
|---|---|---|---|
| Baseline DRV Phenotype | All | De Novo ENF | Re-Used/ No ENF |
| Overall | 175/417 (42%) | 61/112 (54%) | 131/327 (40%) |
| 0 – 7 | 148/270 (55%) | 44/65 (68%) | 104/205 (51%) |
| > 7 – 20 | 16/53 (30%) | 7/17 (41%) | 9/36 (25%) |
| > 20 | 11/94 (12%) | 6/23 (26%) | 5/71 (7%) |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Darunavir was evaluated for carcinogenic potential by oral gavage administration to mice and rats up to 104 weeks. Daily doses of 150, 450 and 1000 mg/kg were administered to mice and doses of 50, 150 and 500 mg/kg was administered to rats. A dose-related increase in the incidence of hepatocellular adenomas and carcinomas were observed in males and females of both species as well as an increase in thyroid follicular cell adenomas in male rats. The observed hepatocellular findings in rodents are considered to be of limited relevance to humans. Repeated administration of darunavir to rats caused hepatic microsomal enzyme induction and increased thyroid hormone elimination, which predispose rats, but not humans, to thyroid neoplasms. At the highest tested doses, the systemic exposures to darunavir (based on AUC) were between 0.4- and 0.7-fold (mice) and 0.7- and 1-fold (rats), relative to those observed in humans at the recommended therapeutic doses (600/100 mg twice daily or 800/100 mg once daily).
Darunavir was not mutagenic or genotoxic in a battery of in vitro and in vivo assays including bacterial reserve mutation (Ames), chromosomal aberration in human lymphocytes and in vivo micronucleus test in mice.
13.2 Animal Toxicology and/or Pharmacology
In juvenile rats single doses of darunavir (20 mg/kg to 160 mg/kg at ages 5–11 days) or multiple doses of darunavir (40 mg/kg to 1000 mg/kg at age 12 days) caused mortality. The mortalities were associated with convulsions in some of the animals. Within this age range exposures in plasma, liver and brain were dose and age dependent and were considerably greater than those observed in adult rats. These findings were attributed to the ontogeny of the CYP450 liver enzymes involved in the metabolism of darunavir and the immaturity of the blood-brain barrier. No treatment-related mortalities were noted in juvenile rats after a single dose of darunavir at 1000 mg/kg on day 26 of age or after repeat dosing at 500 mg/kg from day 23 to 50 of age. The exposures and toxicity profile in the older animals (day 23 or day 26) were comparable to those observed in adult rats. Due to uncertainties regarding the rate of development of the human blood-brain barrier and liver enzymes, do not administer PREZISTA/ritonavir in pediatric patients below 3 years of age.
14 CLINICAL STUDIES
14.1 Description of Adult Clinical Studies
The evidence of efficacy of PREZISTA/ritonavir is based on the analyses of 192-week data from a randomized, controlled open-label Phase 3 trial in treatment-naïve (TMC114-C211) HIV-1-infected adult subjects and 96-week data from a randomized, controlled, open-label Phase 3 trial in antiretroviral treatment-experienced (TMC114-C214) HIV-1-infected adult subjects. In addition, 96-week data are included from 2 randomized, controlled Phase 2b trials, TMC114-C213 and TMC114-C202, in antiretroviral treatment-experienced HIV-1-infected adult subjects.
14.2 Treatment-Naïve Adult Subjects
Study TMC114-C211
Study TMC114-C211 is a randomized, controlled, open-label Phase 3 trial comparing PREZISTA/ritonavir 800/100 mg once daily versus lopinavir/ritonavir 800/200 mg per day (given as a twice daily or as a once daily regimen) in antiretroviral treatment-naïve HIV-1-infected adult subjects. Both arms used a fixed background regimen consisting of tenofovir disoproxil fumarate 300 mg once daily (TDF) and emtricitabine 200 mg once daily (FTC).
HIV-1-infected subjects who were eligible for this trial had plasma HIV-1 RNA greater than or equal to 5000 copies/mL. Randomization was stratified by screening plasma viral load (HIV-1 RNA less than 100,000 copies/mL or greater than or equal to 100,000 copies/mL) and screening CD4+ cell count (less than 200 cells/mm3 or greater than or equal to 200 cells/mm3). Virologic response was defined as a confirmed plasma HIV-1 RNA viral load less than 50 copies/mL. Analyses included 689 subjects in Study TMC114-C211 who had completed 192 weeks of treatment or discontinued earlier.
Demographics and baseline characteristics were balanced between the PREZISTA/ritonavir arm and the lopinavir/ritonavir arm (see Table 16). Table 16 compares the demographic and baseline characteristics between subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and subjects in the lopinavir/ritonavir 800/200 mg per day arm in Study TMC114-C211.
| Randomized Study TMC114-C211 | ||
|---|---|---|
| PREZISTA/ritonavir 800/100 mg once daily + TDF/FTC N = 343 | lopinavir/ritonavir 800/200 mg per day + TDF/FTC N = 346 |
|
| Demographic Characteristics | ||
| Median Age (years) (range, years) | 34 (18–70) | 33 (19–68) |
| Sex | ||
| Male | 70% | 70% |
| Female | 30% | 30% |
| Race | ||
| White | 40% | 45% |
| Black | 23% | 21% |
| Hispanic | 23% | 22% |
| Asian | 13% | 11% |
| Baseline Characteristics | ||
| Mean Baseline Plasma HIV-1 RNA (log10 copies/mL) | 4.86 | 4.84 |
| Median Baseline CD4+ Cell Count (cells/mm3) (range, cells/mm3) | 228 (4–750) | 218 (2–714) |
| Percentage of Patients with Baseline Viral Load ≥ 100,000 copies/mL | 34% | 35% |
| Percentage of Patients with Baseline CD4+ Cell Count < 200 cells/mm3 | 41% | 43% |
Week 192 outcomes for subjects on PREZISTA/ritonavir 800/100 mg once daily from Study TMC114-C211 are shown in Table 17.
| PREZISTA/ritonavir 800/100 mg once daily + TDF/FTC N = 343 | lopinavir/ritonavir 800/200 mg per day + TDF/FTC N = 346 |
|
|---|---|---|
| N = total number of subjects with data | ||
|
||
| Virologic success HIV-1 RNA < 50 copies/mL | 70%* | 61% |
| Virologic failure† | 12% | 15% |
| No virologic data at Week 192 window‡
Reasons | ||
| Discontinued study due to adverse event or death§ | 5% | 13% |
| Discontinued study for other reasons¶ | 13% | 12% |
| Missing data during window‡ but on study | <1% | 0% |
In Study TMC114-C211 at 192 weeks of treatment, the median increase from baseline in CD4+ cell counts was 258 cells/mm3 in the PREZISTA/ritonavir 800/100 mg once daily arm and 263 cells/mm3 in the lopinavir/ritonavir 800/200 mg per day arm. Of the PREZISTA/ritonavir subjects with a confirmed virologic response of < 50 copies/mL at Week 48, 81% remained undetectable at Week 192 versus 68% with lopinavir/ritonavir. In the 192 week analysis, statistical superiority of the PREZISTA/ritonavir regimen over the lopinavir/ritonavir regimen was demonstrated for both ITT and OP populations.
14.3 Treatment-Experienced Adult Subjects
Study TMC114-C229
Study TMC114-C229 is a randomized, open-label trial comparing PREZISTA/ritonavir 800/100 mg once daily to PREZISTA/ritonavir 600/100 mg twice daily in treatment-experienced HIV-1-infected patients with screening genotype resistance test showing no darunavir resistance associated substitutions (i.e. V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V, L89V) and a screening viral load of greater than 1,000 HIV-1 RNA copies/mL. Both arms used an optimized background regimen consisting of greater than or equal to 2 NRTIs selected by the investigator.
HIV-1-infected subjects who were eligible for this trial were on a highly active antiretroviral therapy regimen (HAART) for at least 12 weeks. Virologic response was defined as a confirmed plasma HIV-1 RNA viral load less than 50 copies/mL. Analyses included 590 subjects who had completed 48 weeks of treatment or discontinued earlier.
Table 18 compares the demographic and baseline characteristics between subjects in the PREZISTA/ritonavir 800/100 mg once daily arm and subjects in the PREZISTA/ritonavir 600/100 mg twice daily arm in Study TMC114-C229. No imbalances between the 2 arms were noted.
| Randomized Study TMC114-C229 | ||
|---|---|---|
| PREZISTA/ritonavir 800/100 mg once daily + OBR N = 294 | PREZISTA/ritonavir 600/100 mg twice daily + OBR N = 296 |
|
| Demographic Characteristics | ||
| Median Age (years) (range, years) | 40 (18–70) | 40 (18–77) |
| Sex | ||
| Male | 61% | 67% |
| Female | 39% | 33% |
| Race | ||
| White | 35% | 37% |
| Black | 28% | 24% |
| Hispanic | 16% | 20% |
| Asian | 16% | 14% |
| Baseline Characteristics | ||
| Mean Baseline Plasma HIV-1 RNA (log10 copies/mL) | 4.19 | 4.13 |
| Median Baseline CD4+ Cell Count (cells/mm3) (range, cells/mm3) | 219 (24–1306) | 236 (44–864) |
| Percentage of Patients with Baseline Viral Load ≥ 100,000 copies/mL | 13% | 11% |
| Percentage of Patients with Baseline CD4+ Cell Count < 200 cells/mm3 | 43% | 39% |
| Median Darunavir Fold Change (range)* | 0.50 (0.1–1.8) | 0.50 (0.1–1.9) |
| Median Number of Resistance-Associated† : | ||
| PI mutations | 3 | 4 |
| NNRTI mutations | 2 | 1 |
| NRTI mutations | 1 | 1 |
| Percentage of Subjects Susceptible to All Available PIs at Baseline | 88% | 86% |
| Percentage of Subjects with Number of Baseline Primary Protease Inhibitor Mutations† : | ||
| 0 | 84% | 84% |
| 1 | 8% | 9% |
| 2 | 5% | 4% |
| ≥ 3 | 3% | 2% |
| Median Number of ARVs Previously Used‡: | ||
| NRTIs | 3 | 3 |
| NNRTIs | 1 | 1 |
| PIs (excluding low-dose ritonavir) | 1 | 1 |
Week 48 outcomes for subjects on PREZISTA/ritonavir 800/100 mg once daily from Study TMC114-C229 are shown in Table 19.
| Randomized Study TMC114-C229 | ||
|---|---|---|
| PREZISTA/ritonavir 800/100 mg once daily + OBR N = 294 | PREZISTA/ritonavir 600/100 mg twice daily + OBR N = 296 |
|
| N = total number of subjects with data | ||
|
||
| Virologic success HIV-1 RNA < 50 copies/mL | 69% | 69% |
| Virologic failure* | 26% | 23% |
| No virologic data at Week 48 window†
Reasons | ||
| Discontinued study due to adverse event or death‡ | 3% | 4% |
| Discontinued study for other reasons§ | 2% | 3% |
| Missing data during window† but on study | 0% | < 1% |
The mean increase from baseline in CD4+ cell counts was comparable for both treatment arms (108 cells/mm3 and 112 cells/mm3 in the PREZISTA/ritonavir 800/100 mg once daily arm and the PREZISTA/ritonavir 600/100 mg twice daily arm, respectively).
Study TMC114-C214
Study TMC114-C214 is a randomized, controlled, open-label Phase 3 trial comparing PREZISTA/ritonavir 600/100 mg twice daily versus lopinavir/ritonavir 400/100 mg twice daily in antiretroviral treatment-experienced, lopinavir/ritonavir-naïve HIV-1-infected adult subjects. Both arms used an optimized background regimen (OBR) consisting of at least 2 antiretrovirals (NRTIs with or without NNRTIs).
HIV-1-infected subjects who were eligible for this trial had plasma HIV-1 RNA greater than 1000 copies/mL and were on a highly active antiretroviral therapy regimen (HAART) for at least 12 weeks. Virologic response was defined as a confirmed plasma HIV-1 RNA viral load less than 400 copies/mL. Analyses included 595 subjects in Study TMC114-C214 who had completed 96 weeks of treatment or discontinued earlier.
Demographics and baseline characteristics were balanced between the PREZISTA/ritonavir arm and the lopinavir/ritonavir arm (see Table 20). Table 20 compares the demographic and baseline characteristics between subjects in the PREZISTA/ritonavir 600/100 mg twice daily arm and subjects in the lopinavir/ritonavir 400/100 mg twice daily arm in Study TMC114-C214.
| Randomized Study TMC114-C214 | ||
|---|---|---|
| PREZISTA/ritonavir 600/100 mg twice daily + OBR N = 298 | lopinavir/ritonavir 400/100 mg twice daily + OBR N = 297 |
|
| Demographic Characteristics | ||
| Median Age (years) (range, years) | 40 (18–68) | 41 (22–76) |
| Sex | ||
| Male | 77% | 81% |
| Female | 23% | 19% |
| Race | ||
| White | 54% | 57% |
| Black | 18% | 17% |
| Hispanic | 15% | 15% |
| Asian | 9% | 9% |
| Baseline Characteristics | ||
| Mean Baseline Plasma HIV-1 RNA (log10 copies/mL) | 4.33 | 4.28 |
| Median Baseline CD4+ Cell Count (cells/mm3) (range, cells/mm3) | 235 (3–831) | 230 (2–1096) |
| Percentage of Patients with Baseline Viral Load ≥ 100,000 copies/mL | 19% | 17% |
| Percentage of Patients with Baseline CD4+ Cell Count < 200 cells/mm3 | 40% | 40% |
| Median Darunavir Fold Change (range) | 0.60 (0.10–37.40) | 0.60 (0.1–43.8) |
| Median Lopinavir Fold Change (range) | 0.70 (0.40–74.40) | 0.80 (0.30–74.50) |
| Median Number of Resistance-Associated*: | ||
| PI mutations | 4 | 4 |
| NNRTI mutations | 1 | 1 |
| NRTI mutations | 2 | 2 |
| Percentage of Subjects with Number of Baseline Primary Protease Inhibitor Mutations*: | ||
| ≤ 1 | 78% | 80% |
| 2 | 8% | 9% |
| ≥ 3 | 13% | 11% |
| Median Number of ARVs Previously Used†: | ||
| NRTIs | 4 | 4 |
| NNRTIs | 1 | 1 |
| PIs (excluding low-dose ritonavir) | 1 | 1 |
| Percentage of Subjects Resistant‡ to All Available§ PIs at Baseline, excluding Darunavir | 2% | 3% |
Week 96 outcomes for subjects on PREZISTA/ritonavir 600/100 mg twice daily from Study TMC114-C214 are shown in Table 21.
| PREZISTA/ritonavir 600/100 mg twice daily + OBR N = 298 | lopinavir/ritonavir 400/100 mg twice daily + OBR N = 297 |
|
|---|---|---|
| N = total number of subjects with data | ||
|
||
| Virologic success HIV-1 RNA < 50 copies/mL | 58% | 52% |
| Virologic failure* | 26% | 33% |
| No virologic data at Week 96 window†
Reasons | ||
| Discontinued study due to adverse event or death‡ | 7% | 8% |
| Discontinued study for other reasons§ | 8% | 7% |
| Missing data during window† but on study | 1% | < 1% |
In Study TMC114-C214 at 96 weeks of treatment, the median increase from baseline in CD4+ cell counts was 81 cells/mm3 in the PREZISTA/ritonavir 600/100 mg twice daily arm and 93 cells/mm3 in the lopinavir/ritonavir 400/100 mg twice daily arm.
Studies TMC114-C213 and TMC114-C202
Studies TMC114-C213 and TMC114-C202 are randomized, controlled, Phase 2b trials in adult subjects with a high level of PI resistance consisting of 2 parts: an initial partially-blinded, dose-finding part and a second long-term part in which all subjects randomized to PREZISTA/ritonavir received the recommended dose of 600/100 mg twice daily.
HIV-1-infected subjects who were eligible for these trials had plasma HIV-1 RNA greater than 1000 copies/mL, had prior treatment with PI(s), NNRTI(s) and NRTI(s), had at least one primary PI mutation (D30N, M46I/L, G48V, I50L/V, V82A/F/S/T, I84V, L90M) at screening, and were on a stable PI-containing regimen at screening for at least 8 weeks. Randomization was stratified by the number of PI mutations, screening viral load, and the use of enfuvirtide.
The virologic response rate was evaluated in subjects receiving PREZISTA/ritonavir plus an OBR versus a control group receiving an investigator-selected PI(s) regimen plus an OBR. Prior to randomization, PI(s) and OBR were selected by the investigator based on genotypic resistance testing and prior ARV history. The OBR consisted of at least 2 NRTIs with or without enfuvirtide. Selected PI(s) in the control arm included: lopinavir in 36%, (fos)amprenavir in 34%, saquinavir in 35% and atazanavir in 17%; 98% of control subjects received a ritonavir boosted PI regimen out of which 23% of control subjects used dual-boosted PIs. Approximately 47% of all subjects used enfuvirtide, and 35% of the use was in subjects who were ENF-naïve. Virologic response was defined as a decrease in plasma HIV-1 RNA viral load of at least 1 log10 versus baseline.
In the pooled analysis for TMC114-C213 and TMC114-C202, demographics and baseline characteristics were balanced between the PREZISTA/ritonavir arm and the comparator PI arm (see Table 22). Table 22 compares the demographic and baseline characteristics between subjects in the PREZISTA/ritonavir 600/100 mg twice daily arm and subjects in the comparator PI arm in the pooled analysis of Studies TMC114-C213 and TMC114-C202.
| Randomized Studies TMC114-C213 and TMC114-C202 |
||
|---|---|---|
| PREZISTA/ritonavir 600/100 mg twice daily + OBR N = 131 | Comparator PI(s) + OBR N = 124 |
|
| Demographic Characteristics | ||
| Median Age (years) (range, years) | 43 (27–73) | 44 (25–65) |
| Sex | ||
| Male | 89% | 88% |
| Female | 11% | 12% |
| Race | ||
| White | 81% | 73% |
| Black | 10% | 15% |
| Hispanic | 7% | 8% |
| Baseline Characteristics | ||
| Mean Baseline Plasma HIV-1 RNA (log10 copies/mL) | 4.61 | 4.49 |
| Median Baseline CD4+ Cell Count (cells/mm3) (range, cells/mm3) | 153 (3–776) | 163 (3–1274) |
| Percentage of Patients with Baseline Viral Load > 100,000 copies/mL | 24% | 29% |
| Percentage of Patients with Baseline CD4+ Cell Count < 200 cells/mm3 | 67% | 58% |
| Median Darunavir Fold Change | 4.3 | 3.3 |
| Median Number of Resistance-Associated*: | ||
| PI mutations | 12 | 12 |
| NNRTI mutations | 1 | 1 |
| NRTI mutations | 5 | 5 |
| Percentage of Subjects with Number of Baseline Primary Protease Inhibitor Mutations*: | ||
| ≤ 1 | 8% | 9% |
| 2 | 22% | 21% |
| ≥ 3 | 70% | 70% |
| Median Number of ARVs Previously Used†: | ||
| NRTIs | 6 | 6 |
| NNRTIs | 1 | 1 |
| PIs (excluding low-dose ritonavir) | 5 | 5 |
| Percentage of Subjects Resistant† to All Available‡ PIs at Baseline, excluding Tipranavir and Darunavir | 63% | 61% |
| Percentage of Subjects with Prior Use of Enfuvirtide | 20% | 17% |
Week 96 outcomes for subjects on the recommended dose PREZISTA/ritonavir 600/100 mg twice daily from the pooled Studies TMC114-C213 and TMC114-C202 are shown in Table 23.
| Randomized Studies TMC114-C213 and TMC114-C202 |
||
|---|---|---|
| PREZISTA/ritonavir 600/100 mg twice daily + OBR N=131 | Comparator PI(s) + OBR N=124 |
|
|
||
| Virologic Responders confirmed at least 1 log10 HIV-1 RNA below baseline through Week 96 (< 50 copies/mL at Week 96) | 57% (39%) | 10% (9%) |
| Virologic failures | 29% | 80% |
| Lack of initial response* | 8% | 53% |
| Rebounder† | 17% | 19% |
| Never Suppressed‡ | 4% | 8% |
| Death or discontinuation due to adverse events | 9% | 3% |
| Discontinuation due to other reasons | 5% | 7% |
In the pooled Studies TMC114-C213 and TMC114-C202 through 48 weeks of treatment, the proportion of subjects with HIV-1 RNA less than 400 copies/mL in the arm receiving PREZISTA/ritonavir 600/100 mg twice daily compared to the comparator PI arm was 55.0% and 14.5%, respectively. In addition, the mean changes in plasma HIV-1 RNA from baseline were –1.69 log10 copies/mL in the arm receiving PREZISTA/ritonavir 600/100 mg twice daily and –0.37 log10 copies/mL for the comparator PI arm. The mean increase from baseline in CD4+ cell counts was higher in the arm receiving PREZISTA/ritonavir 600/100 mg twice daily (103 cells/mm3) than in the comparator PI arm (17 cells/mm3).
14.4 Pediatric Patients
The pharmacokinetic profile, safety and antiviral activity of PREZISTA/ritonavir were evaluated in 2 randomized, open-label, multicenter studies.
Study TMC114-C212
Treatment-experienced pediatric subjects between the ages of 6 and less than 18 years and weighing at least 20 kg were stratified according to their weight (greater than or equal to 20 kg to less than 30 kg, greater than or equal to 30 kg to less than 40 kg, greater than or equal to 40 kg) and received PREZISTA tablets with either ritonavir capsules or oral solution plus background therapy consisting of at least two non-protease inhibitor antiretroviral drugs. Eighty patients were randomized and received at least one dose of PREZISTA/ritonavir. Pediatric subjects who were at risk of discontinuing therapy due to intolerance of ritonavir oral solution (e.g., taste aversion) were allowed to switch to the capsule formulation. Of the 44 pediatric subjects taking ritonavir oral solution, 23 subjects switched to the 100 mg capsule formulation and exceeded the weight-based ritonavir dose without changes in observed safety.
The 80 randomized pediatric subjects had a median age of 14 (range 6 to less than 18 years), and were 71% male, 54% Caucasian, 30% Black, 9% Hispanic and 8% other. The mean baseline plasma HIV-1 RNA was 4.64 log10 copies/mL, and the median baseline CD4+ cell count was 330 cells/mm3 (range: 6 to 1505 cells/mm3). Overall, 38% of pediatric subjects had baseline plasma HIV-1 RNA ≥ 100,000 copies/mL. Most pediatric subjects (79%) had previous use of at least one NNRTI and 96% of pediatric subjects had previously used at least one PI.
Seventy-seven pediatric subjects (96%) completed the 24-week period. Of the patients who discontinued, one patient discontinued treatment due to an adverse event. An additional 2 patients discontinued for other reasons, one patient due to compliance and another patient due to relocation.
The proportion of pediatric subjects with HIV-1 RNA less than 400 copies/mL and less than 50 copies/mL was 64% and 50%, respectively. The mean CD4+ cell count increase from baseline was 117 cells/mm3.
Study TMC114-C228
Treatment-experienced pediatric subjects between the ages of 3 and less than 6 years and weighing greater than or equal to 10 kg to less than 20 kg received PREZISTA oral suspension with ritonavir oral solution plus background therapy consisting of at least two active non-protease inhibitor antiretroviral drugs. Twenty-one subjects received at least one dose of PREZISTA/ritonavir.
The 21 subjects had a median age of 4.4 years (range 3 to less than 6 years), and were 48% male, 57% Black, 29%, Caucasian and 14% other. The mean baseline plasma HIV-1 was 4.34 log10 copies/mL, the median baseline CD4+ cell count was 927 × 106 cells/l (range: 209 to 2,429 × 106 cells/l) and the median baseline CD4+ percentage was 27.7% (range: 15.6% to 51.1%). Overall, 24% of subjects had a baseline plasma HIV-1 RNA greater than or equal to 100,000 copies/mL. All subjects had used greater than or equal to 2 NRTIs, 62% of subjects had used greater than or equal to 1 NNRTI and 76% had previously used at least one HIV PI.
Twenty subjects (95%) completed the 24 week period. One subject prematurely discontinued treatment due to vomiting assessed as related to ritonavir.
The proportion of subjects with HIV-1 RNA less than 50 copies/mL and less than 400 copies/mL was 57% and 81%, respectively. The mean change in CD4+ percentage from baseline was 4%. The mean change in CD4+ cell count from baseline was 109 × 106 cells/L.
Dose recommendations from the two studies were based on the following:
- Similar darunavir plasma exposures in children compared to adults, and
- Similar virologic response rates and safety profile in children compared to adults
16 HOW SUPPLIED/STORAGE AND HANDLING
PREZISTA (darunavir) 400 mg tablets are supplied as light orange, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 400 mg of darunavir per tablet. Each tablet is debossed with "400MG" on one side and "TMC" on the other side.
PREZISTA (darunavir) 600 mg tablets are supplied as orange, oval-shaped, film-coated tablets containing darunavir ethanolate equivalent to 600 mg of darunavir per tablet. Each tablet is debossed with "600MG" on one side and "TMC" on the other side.
PREZISTA is packaged in bottles in the following configuration:
400 mg tablets—bottles of 60 (NDC 54868-5969-0)
600 mg tablets—bottles of 60 (NDC 54868-6369-0)
Storage:
PREZISTA Oral Suspension
- Store at 25°C (77°F); with excursions permitted to 15°–30°C (59°–86°F).
- Do not refrigerate or freeze. Avoid exposure to excessive heat.
- Store in the original container.
- Shake well before each usage.
PREZISTA Tablets
- Store at 25°C (77°F); with excursions permitted to 15°–30°C (59°–86°F).
17 PATIENT COUNSELING INFORMATION
[See FDA-Approved Patient Labeling (Patient Information)]
A statement to patients and healthcare providers is included on the product's bottle label: ALERT: Find out about medicines that should NOT be taken with PREZISTA. A Patient Package Insert for PREZISTA is available for patient information.
17.1 Information About Therapy with PREZISTA
PREZISTA is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care of a physician when using PREZISTA.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. We do not know if PREZISTA can be passed to the baby through breast milk and whether it could harm the baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
17.2 Instructions for Use
Patients should be advised to take PREZISTA and ritonavir (NORVIR®) with food every day as prescribed. Patients should be instructed to swallow whole tablets with a drink such as water or milk. PREZISTA must always be used with ritonavir (NORVIR®) in combination with other antiretroviral drugs. Patients should not alter the dose of either PREZISTA or ritonavir (NORVIR®), discontinue ritonavir (NORVIR®), or discontinue therapy with PREZISTA without consulting their physician.
Patients Taking PREZISTA Once Daily
If a patient misses a dose of PREZISTA or ritonavir (NORVIR®) by more than 12 hours, the patient should be told to wait and then take the next dose of PREZISTA and ritonavir (NORVIR®) at the regularly scheduled time. If the patient misses a dose of PREZISTA or ritonavir (NORVIR®) by less than 12 hours, the patient should be told to take PREZISTA and ritonavir (NORVIR®) immediately, and then take the next dose of PREZISTA and ritonavir (NORVIR®) at the regularly scheduled time. If a dose of PREZISTA or ritonavir (NORVIR®) is skipped, the patient should not double the next dose. Inform the patient that he or she should not take more or less than the prescribed dose of PREZISTA or ritonavir (NORVIR®).
Patients Taking PREZISTA Twice Daily
If a patient misses a dose of PREZISTA or ritonavir (NORVIR®) by more than 6 hours, the patient should be told to wait and then take the next dose of PREZISTA and ritonavir (NORVIR®) at the regularly scheduled time. If the patient misses a dose of PREZISTA or ritonavir (NORVIR®) by less than 6 hours, the patient should be told to take PREZISTA and ritonavir (NORVIR®) immediately, and then take the next dose of PREZISTA and ritonavir (NORVIR®) at the regularly scheduled time. If a dose of PREZISTA or ritonavir (NORVIR®) is skipped, the patient should not double the next dose. Inform the patient that he or she should not take more or less than the prescribed dose of PREZISTA or ritonavir (NORVIR®).
17.3 Hepatotoxicity
Patients should be informed that Drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) has been reported with PREZISTA co-administered with 100 mg of ritonavir. Monitor liver function before and during therapy, especially in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases. Post-marketing cases of liver injury, including some fatalities, have been reported. Patients should be advised about the signs and symptoms of liver problems. These may include jaundice of the skin or eyes, dark (tea colored) urine, pale colored stools, nausea, vomiting, loss of appetite, or pain, aching or sensitivity in the right upper quadrant of the abdomen.
17.4 Severe Skin Reactions
Patients should be informed that skin reactions ranging from mild to severe, including Stevens-Johnson Syndrome and toxic epidermal necrolysis, have been reported with PREZISTA co-administered with 100 mg of ritonavir. Patients should be advised to discontinue PREZISTA/ritonavir immediately if signs or symptoms of severe skin reactions develop. These can include but are not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis and/or eosinophilia.
17.5 Drug Interactions
PREZISTA/ritonavir may interact with many drugs; therefore, patients should be advised to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, including St. John's wort.
Patients receiving estrogen-based contraceptives should be instructed to use alternate contraceptive measures during therapy with PREZISTA/ritonavir because hormonal levels may decrease.
Manufactured by:
PREZISTA oral suspension
Janssen Pharmaceutica, N.V.
Beerse, Belgium
PREZISTA tablets
Janssen Ortho LLC, Gurabo, PR 00778
Manufactured for:
Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560
NORVIR® is a registered trademark of its respective owner.
PREZISTA® is a registered trademark of Janssen Pharmaceuticals.
© Janssen Pharmaceuticals, Inc. 2006
Distributed by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
Patient Information
PREZISTA (pre-ZIS-ta)
(darunavir)
Oral Suspension
PREZISTA (pre-ZIS-ta)
(darunavir)
Tablets
Read this Patient Information before you start taking PREZISTA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Also read the Patient Information leaflet for NORVIR® (ritonavir).
What is the most important information I should know about PREZISTA?
- PREZISTA can interact with other medicines and cause serious side effects. It is important to know the medicines that should not be taken with PREZISTA. See the section "Who should not take PREZISTA?"
- PREZISTA may cause liver problems. Some people taking PREZISTA in combination with NORVIR® (ritonavir) have developed liver problems which may be life-threatening. Your healthcare provider should do blood tests before and during your combination treatment with PREZISTA. If you have chronic hepatitis B or C infection, your healthcare provider should check your blood tests more often because you have an increased chance of developing liver problems.
- Tell your healthcare provider if you have any of the below signs and symptoms of liver problems.
- Dark (tea colored) urine
- yellowing of your skin or whites of your eyes
- pale colored stools (bowel movements)
- nausea
- vomiting
- pain or tenderness on your right side below your ribs
- loss of appetite
PREZISTA may cause severe or life-threatening skin reactions or rash. Sometimes these skin reactions and skin rashes can become severe and require treatment in a hospital. You should call your healthcare provider immediately if you develop a rash. However, stop taking PREZISTA and ritonavir combination treatment and call your healthcare provider immediately if you develop any skin changes with symptoms below:
- fever
- tiredness
- muscle or joint pain
- blisters or skin lesions
- mouth sores or ulcers
- red or inflamed eyes, like "pink eye" (conjunctivitis)
Rash occurred more often in patients taking PREZISTA and raltegravir together than with either drug separately, but was generally mild.
See "What are the possible side effects of PREZISTA?" for more information about side effects.
What is PREZISTA?
PREZISTA is a prescription anti-HIV medicine used with ritonavir and other anti-HIV medicines to treat adults and children 3 years of age and older with human immunodeficiency virus (HIV-1) infection. PREZISTA is a type of anti-HIV medicine called a protease inhibitor. HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
When used with other HIV medicines, PREZISTA may help to reduce the amount of HIV in your blood (called "viral load"). PREZISTA may also help to increase the number of white blood cells called CD4 (T) cell which help fight off other infections. Reducing the amount of HIV and increasing the CD4 (T) cell count may improve your immune system. This may reduce your risk of death or infections that can happen when your immune system is weak (opportunistic infections).
Children under 3 years of age should not take PREZISTA.
PREZISTA does not cure HIV infection or AIDS and you may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. You should remain under the care of a doctor when using PREZISTA.
Avoid doing things that can spread HIV-1 infection.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
Ask your healthcare provider if you have any questions on how to prevent passing HIV to other people.
Who should not take PREZISTA?
Do not take PREZISTA with any of the following medicines:
- alfuzosin (Uroxatral®)
- dihydroergotamine (D.H.E. 45®, Embolex®, Migranal®), ergonovine, ergotamine (Cafergot®,Ergomar®) methylergonovine
- cisapride
- pimozide (Orap®)
- oral midazolam, triazolam (Halcion®)
- the herbal supplement St. John's Wort (Hypericum perforatum)
- the cholesterol lowering medicines lovastatin (Mevacor®, Altoprev®, Advicor®) or simvastatin (Zocor®, Simcor®, Vytorin®)
- rifampin (Rifadin®, Rifater®, Rifamate®, Rimactane®)
- sildenafil (Revatio®) only when used for the treatment of pulmonary arterial hypertension.
Serious problems can happen if you or your child take any of these medicines with PREZISTA.
What should I tell my doctor before I take PREZISTA?
PREZISTA may not be right for you. Before taking PREZISTA, tell your healthcare provider if you:
- have liver problems, including hepatitis B or hepatitis C
- are allergic to sulfa medicines
- have high blood sugar (diabetes)
- have hemophilia
- are pregnant or planning to become pregnant. It is not known if PREZISTA will harm your unborn baby.
Pregnancy Registry: You and your healthcare provider will need to decide if taking PREZISTA is right for you. If you take PREZISTA while you are pregnant, talk to your healthcare provider about how you can be included in the Antiretroviral Pregnancy Registry. The purpose of the registry is follow the health of you and your baby. - are breastfeeding or plan to breastfeed. Do not breastfeed. We do not know if PREZISTA can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Tell your healthcare provider about all the medicines you take including prescription and nonprescription medicines, vitamins, and herbal supplements. Using PREZISTA and certain other medicines may affect each other causing serious side effects. PREZISTA may affect the way other medicines work and other medicines may affect how PREZISTA works.
Especially tell your healthcare provider if you take:
- medicine to treat HIV
- estrogen-based contraceptives (birth control). PREZISTA might reduce the effectiveness of estrogen-based contraceptives. You must take additional precautions for birth control such as a condom.
- medicine for your heart such as bepridil, lidocaine (Xylocaine Viscous®), quinidine (Nuedexta®) , amiodarone (Pacerone®, Cardarone®), digoxin (Lanoxin®), flecainide (Tambocor®), propafenone (Rythmol®)
- warfarin (Coumadin®, Jantoven®)
- medicine for seizures such as carbamazepine (Carbatrol®, Equetro®, Tegretol®, Epitol®), phenobarbital, phenytoin (Dilantin®, Phenytek®)
- medicine for depression such as trazadone and desipramine (Norpramin®)
- clarithromycin (Prevpac®, Biaxin®)
- medicine for fungal infections such as ketoconazole (Nizoral®), itraconazole (Sporanox®, Onmel®), voriconazole (VFend®)
- colchicine (Colcrys®, Col-Probenecid®)
- rifabutin (Mycobutin®)
- medicine used to treat blood pressure, a heart attack, heart failure, or to lower pressure in the eye such as metoprolol (Lopressor®, Toprol-XL®), timolol (Cosopt®, Betimol®, Timoptic®, Isatolol®, Combigan®)
- midazolam administered by injection
- medicine for heart disease such as felodipine (Plendil®), nifedipine
(Procardia®, Adalat CC®, Afeditab CR®), nicardipine (Cardene®) - steroids such as dexamethasone, fluticasone (Advair Diskus®, Veramyst®, Flovent®, Flonase®)
- bosentan(Tracleer®)
- medicine to treat chronic hepatitis C such as boceprevir (Victrelis™), telaprevir (Incivek™)
- medicine for cholesterol such as pravastatin (Pravachol®), atorvastatin (Lipitor®), rosuvastatin (Crestor®)
- medicine to prevent organ transplant failure such as cyclosporine
(Gengraf®, Sandimmune®, Neoral®), tacrolimus (Prograf®), sirolimus (Rapamune®) - salmeterol (Advair®, Serevent®)
- medicine for narcotic withdrawal such as methadone (Methadose®, Dolophine Hydrochloride), buprenorphine (Butrans®, Buprenex®, Subutex®), buprenorphine/naloxone (Suboxone®)
- medicine to treat schizophrenia such as risperidone (Risperdal®), thioridazine
- medicine to treat erectile dysfunction or pulmonary hypertension such as sildenafil ( Viagra®, Revatio®), vardenafil ( Levitra®, Staxyn®), tadalafil (Cialis®, Adcirca®)
- medicine to treat anxiety, depression or panic disorder such as sertraline (Zoloft®), paroxetine (Paxil®)
This is not a complete list of medicines that you should tell your healthcare provider that you are taking. Ask your healthcare provider or pharmacist if you are not sure if your medicine is one that is listed above. Know the medicines you take. Keep a list of them to show your doctor or pharmacist when you get a new medicine. Do not start any new medicines while you are taking PREZISTA without first talking with your healthcare provider.
How should I take PREZISTA?
- Take PREZISTA every day exactly as prescribed by your healthcare provider.
- You must take ritonavir (NORVIR®) at the same time as PREZISTA.
- Do not change your dose of PREZISTA or stop treatment without talking to your healthcare provider first.
- Take PREZISTA and ritonavir (NORVIR®) with food.
- Swallow PREZISTA tablets whole with a drink. If you have difficulty swallowing PREZISTA tablets, PREZISTA oral suspension is also available. Your health care provider will help determine whether PREZISTA tablets or oral suspension is right for you.
- PREZISTA oral suspension should be given with the supplied oral dosing syringe. Shake the suspension well before each usage.
- If your child is taking PREZISTA, your child's healthcare provider will decide the right dose based on your child's weight. Your child's healthcare provider will tell you how much PREZISTA (tablets or oral suspension) and how much ritonavir (NORVIR®) (capsules, tablets or solution) your child should take. Your child should take PREZISTA with ritonavir twice a day with food. If your child does not tolerate ritonavir oral solution, ask your child's healthcare provider for advice.
- If you take too much PREZISTA, call your healthcare provider or go to the nearest hosptial emergency room right away.
What should I do if I miss a dose?
People who take PREZISTA one time a day:
- If you miss a dose of PREZISTA by less than 12 hours, take your missed dose of PREZISTA right away. Then take your next dose of PREZISTA at your regularly scheduled time.
- If you miss a dose of PREZISTA by more than 12 hours, wait and then take the next dose of PREZISTA at your regularly scheduled time.
People who take PREZISTA two times a day
- If you miss a dose of PREZISTA by less than 6 hours, take your missed dose of PREZISTA right away. Then take your next dose of PREZISTA at your regularly scheduled time.
- If you miss a dose of PREZISTA by more than 6 hours, wait and then take the next dose of PREZISTA at your regularly scheduled time.
If a dose of PREZISTA is skipped, do not double the next dose. Do not take more or less than your prescribed dose of PREZISTA at any one time.
What are the possible side effects of PREZISTA?
PREZISTA can cause side effects including:
- See "What is the most important information I should know about PREZISTA?"
- Diabetes and high blood sugar (hyperglycemia). Some people who take protease inhibitors including PREZISTA can get high blood sugar, develop diabetes, or your diabetes can get worse. Tell your healthcare provider if you notice an increase in thirst or urinate often while taking PREZISTA.
- Changes in body fat. These changes can happen in people who take antiretroviral therapy. The changes may include an increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the back, chest, and stomach area. Loss of fat from the legs, arms, and face may also happen. The exact cause and long-term health effects of these conditions are not known.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Call your healthcare provider right away if you start having new symptoms after starting your HIV medicine.
- Increased bleeding for hemophiliacs. Some people with hemophilia have increased bleeding with protease inhibitors including PREZISTA.
The most common side effects of PREZISTA include:
|
|
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of PREZISTA. For more information, ask your health care provider.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store PREZISTA?
- Store PREZISTA oral suspension and tablets at room temperature [77°F (25°C)].
- Do not refrigerate or freeze PREZISTA oral suspension.
- Keep PREZISTA away from high heat.
- PREZISTA oral suspension should be stored in the original container.
Keep PREZISTA and all medicines out of the reach of children.
General information about PREZISTA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use PREZISTA for a condition for which it was not prescribed. Do not give PREZISTA to other people even if they have the same condition you have. It may harm them.
This leaflet summarizes the most important information about PREZISTA. If you would like more information, talk to your healthcare provider. You can ask your healthcare provider or pharmacist for information about PREZISTA that is written for health professionals.
For more information, call 1-800-526-7736.
What are the ingredients in PREZISTA?
Active ingredient: darunavir
Inactive ingredients:
PREZISTA Oral Suspension: hydroxypropyl cellulose, microcrystalline cellulose, sodium carboxymethylcellulose, methylparaben sodium, citric acid monohydrate, sucralose, masking flavor, strawberry cream flavor,
hydrochloric acid (for pH adjustment), purified water.
PREZISTA 75 mg and 150 mg Tablets: colloidal silicon dioxide,
crospovidone, magnesium stearate, microcrystalline cellulose. The film
coating contains: OPADRY® White (polyethylene glycol 3350,
polyvinyl alcohol-partially hydrolyzed, talc, titanium dioxide).
PREZISTA 400 mg and 600 mg Tablets: colloidal silicon dioxide,
crospovidone, magnesium stearate, microcrystalline cellulose. The film
coating contains: OPADRY® Orange (FD&C Yellow No. 6, polyethylene glycol 3350, polyvinyl alcohol-partially hydrolyzed, talc, titanium dioxide).
This Patient Information has been approved by the U.S Food and Drug Administration.
Manufactured by:
PREZISTA Oral Suspension
Janssen Pharmaceutica, N.V.
Beerse, Belgium
PREZISTA Tablets
Janssen Ortho LLC, Gurabo, PR 00778
Manufactured for:
Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560
NORVIR® is a registered trademark of its respective owner.
PREZISTA® is a registered trademark of Janssen Pharmaceuticals
© Janssen Pharmaceuticals, Inc. 2006
Revised: June 2012
INSTRUCTIONS FOR USE
PREZISTA® (pre-ZIS-ta)
(darunavir)
ORAL SUSPENSION
Be sure that you read, understand, and follow these Instructions for Use so that you measure and take PREZISTA Oral Suspension correctly. Ask your pharmacist or healthcare provider if you are not sure.
Each PREZISTA Oral Suspension carton contains:
|  |
Important information for use:
- Shake PREZISTA Oral Suspension well before each use.
- PREZISTA Oral Suspension should be given with the oral dosing syringe provided to make sure you measure the right amount. The oral dosing syringe provided with your PREZISTA Oral Suspension should not be used for any other medicines.
|
Figure A: Shake the bottle
|
|
|
Figure B: Opening the bottle
|
|
|
Figure C: Inserting the adapter
|
|
|
Figure D: Inserting the syringe
|
|
|
Figure E: Withdrawing the Oral Suspension
|
|
|
Figure F: Removing the syringe
|
|
|
Figure G: Taking the dose of PREZISTA
|
|
|
Figure H: Closing the bottle
|
|
|
Figure I: Rinsing the syringe
|
|
|
Figure J: Putting the syringe back together
|
How should I store PREZISTA?
- Store PREZISTA Oral Suspension and the oral dosing syringe at room temperature 77°F (25°C).
- Do not refrigerate or freeze PREZISTA Oral Suspension.
- Keep PREZISTA Oral Suspension away from high heat.
- Store PREZISTA Oral Suspension in the original container.
Keep PREZISTA and all medicines out of the reach of children.
This Instruction for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Janssen Pharmaceutica, N.V.
Beerse, Belgium
Manufactured for:
Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560
© Janssen Pharmaceuticals, Inc. 2006
Issued December 2011