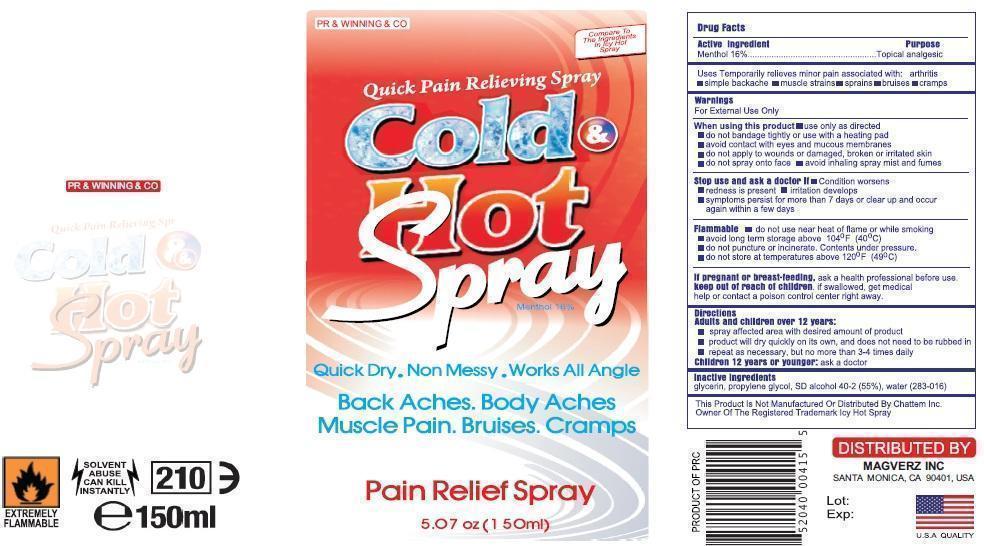
Uses
Temporarily relieves minor pain associated with:
arthritissimple
backachemuscle
strainss
prains
bruise
scramps
Directions
Spray affected are with desired amount of product
Product will dry quickly on its own and doest not to be rubbed in
Repeat as necessary but not more than 3-4 times dilay
Warnings
For External use only
When using this product
Use only as directed
Do not bandage tightly or with heating pad
Avoid contact with eyes and mucous membranes
Do not apply to wounds or damagged, broken or irritated skin
Do not spray on face
Avoid inhaling spray mist and fumes
Stop use and ask docotor if;
Condition Worsesns
Redness is presnt for more than 7 days
Irritation developes
Symptomps persist for mor than 7 dyas or clear up and occur again within a few days.
Flamalble
Do not use near heat or flame or while smoking.
Avod long term storage above 104oF (400C)
Do not puncture of or incinerate.Congents under pressure.
Do not store at temreture above 120oF (490C)
If pregnant or breast feeding
Ask health professionals before use.
If sollowed get medical help or contact poison control center right away.