Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Shake well and gently squeeze the lightweight formula onto fingertips.
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
-
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:- Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Children under 6 months: Ask a doctor.
Inactive Ingredients
Water, Dimethicone, Cyclopentasiloxane, Phenyl Trimethicone, Butylene Glycol, Coco-Caprylate/Caprate, C12-15 Alkyl Benzoate, Caprylic/Capric Triglyceride, Cetyl PEG/PPG-10/1 Dimethicone, Polymethylsilsesquioxane, HDI/Trimethylol Hexyllactone Crosspolymer, Polysilicone-11, PEG/ PPG-18/18 Dimethicone, Honey Extract, Oligopeptide-10, Tetrapeptide- 16, Boswellia Serrata Water, Hydrolyzed Rhodophyceae Extract, Oryza Sativa (Rice) Bran Extract, Vaccinium Angustifolium (Blueberry) Fruit Extract, Ectoin, Carnosine, Hydroxyacetophenone, Butyrospermum Parkii (Shea) Butter, Biosaccharide Gum-4, Alumina, Bis-Ethylhexyl Hydroxydimethoxy Benzylmalonate, Cyclohexasiloxane, Disteardimonium Hectorite, Isopropyl Titanium Triisostearate, Isostearic Acid, Lecithin, Methyldihydrojasmonate, Polyglyceryl-3 Polyricinoleate, Polyhydroxystearic Acid, Polysorbate 20, Propanediol, Silica, Sodium Chloride, Stearic Acid, BHT, 1,2-Hexanediol, Caprylyl Glycol, Sodium Benzoate, Phenoxyethanol. May Contain (+/-): Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499).
Principal Display Panel – 50 mL Cream Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Cream/1.5
DERDS01 8500-09-0013
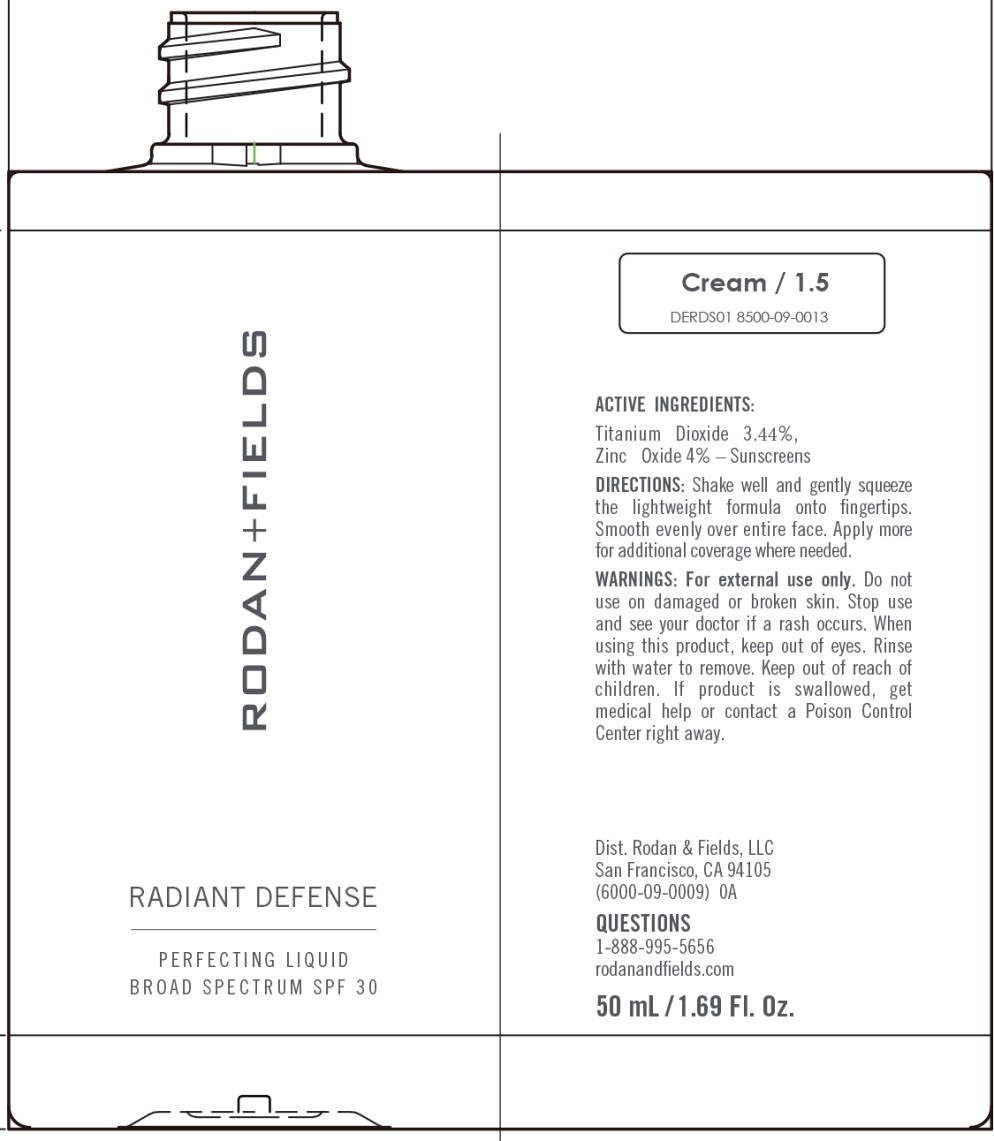
Principal Display Panel – 50 mL Cream Bottle Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Cream/1.5
DERDS01 8500-09-0013
Principal Display Panel – 50 mL Shell Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Shell/1
DERDS01 8500-09-0001
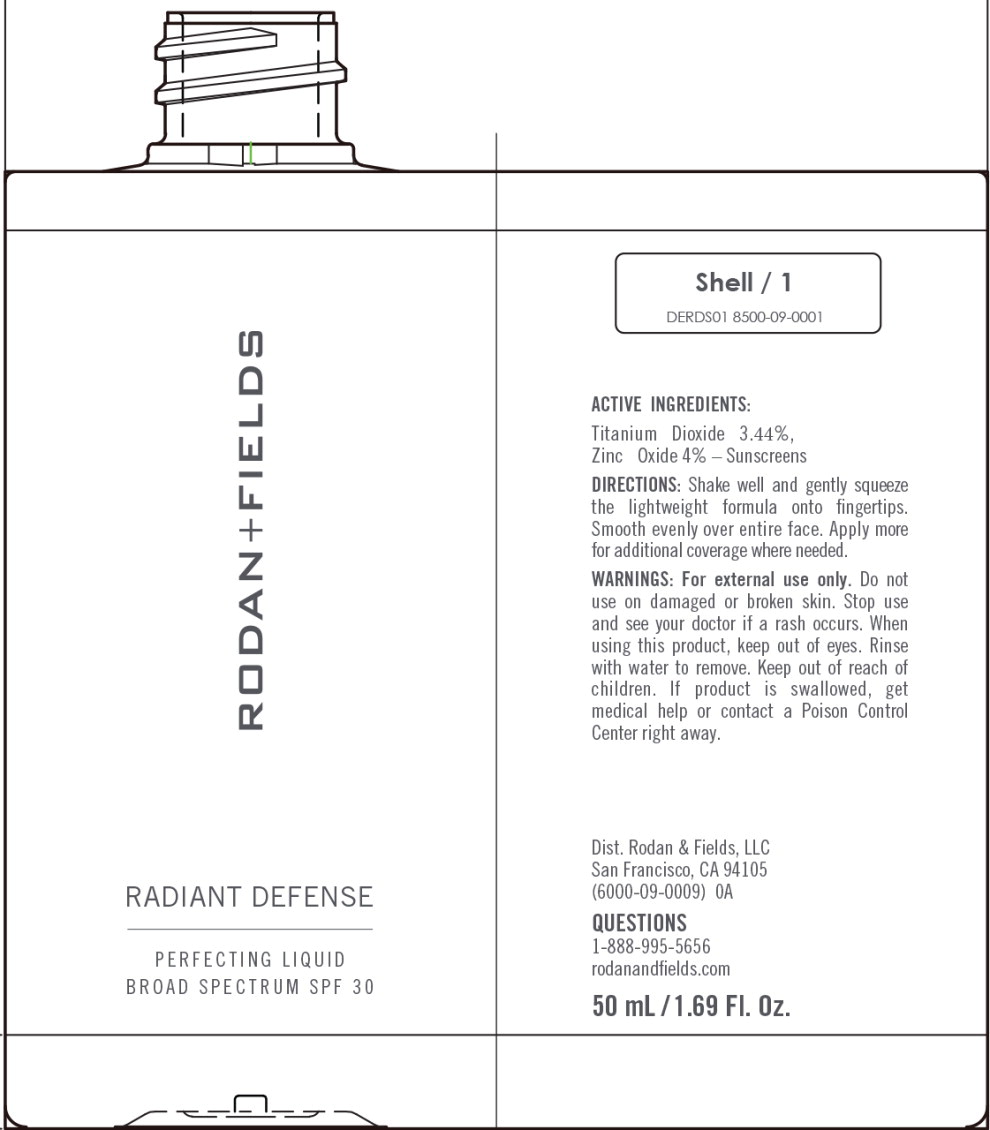
Principal Display Panel – 50 mL Shell Bottle Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Shell/1
DERDS01 8500-09-0001
Principal Display Panel – 50 mL Beige Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Beige/2
DERDS01 8500-09-0002
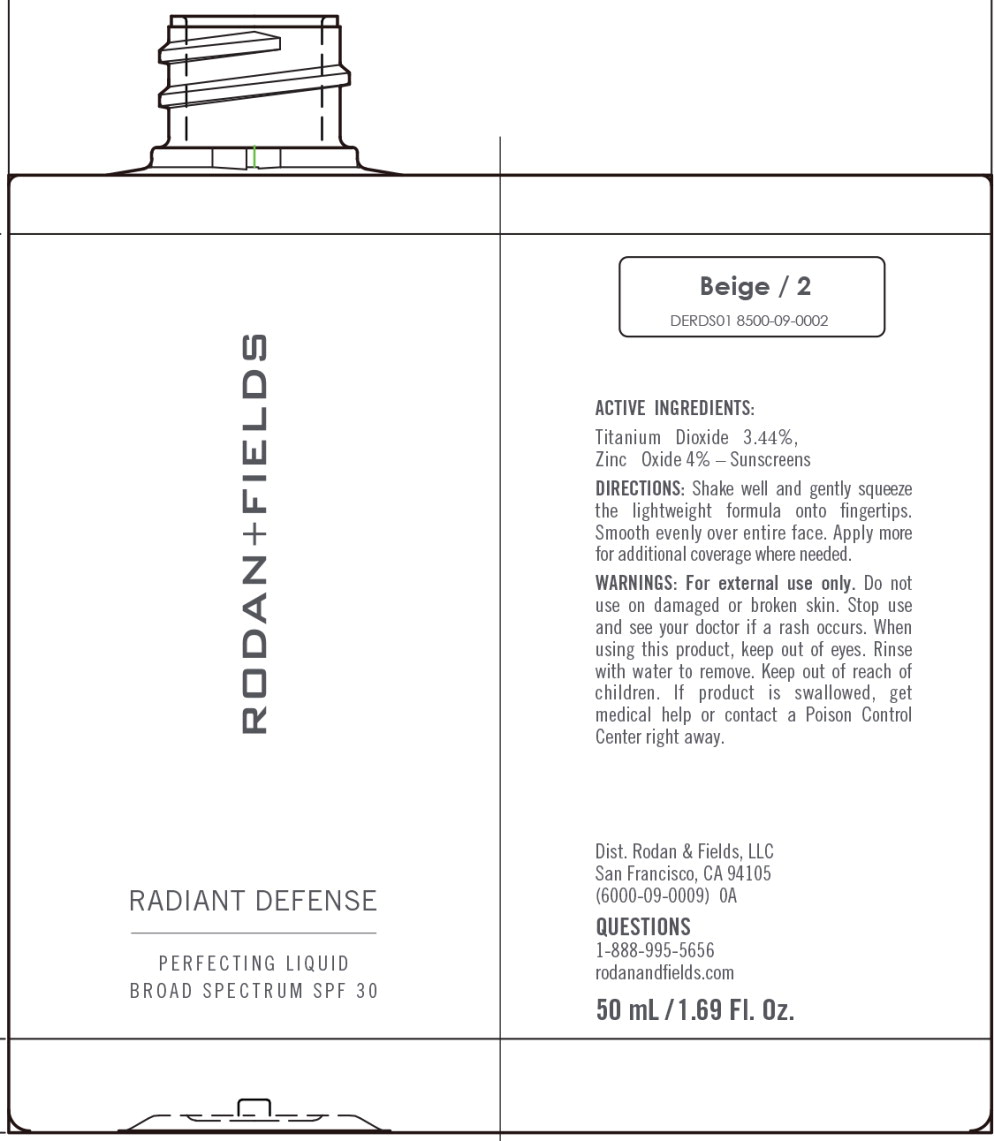
Principal Display Panel – 50 mL Beige Bottle Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Beige/2
DERDS01 8500-09-0002
Principal Display Panel – 50 mL Sand Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Sand/3
DERDS01 8500-09-0003
Principal Display Panel – 50 mL Sand Bottle Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Sand/3
DERDS01 8500-09-0003
Principal Display Panel – 50 mL Honey Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Honey/4.5
DERDS01 8500-09-0014
Principal Display Panel – 50 mL Honey Bottle Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Honey/4.5
DERDS01 8500-09-0014
Principal Display Panel – 50 mL Golden Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Golden/4
DERDS01 8500-09-0004
Principal Display Panel – 50 mL Golden Bottle Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Golden/4
DERDS01 8500-09-000
Principal Display Panel – 50 mL Almond Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Almond/5
DERDS01 8500-09-0004
Principal Display Panel – 50 mL Golden Bottle Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Almond/5
DERDS01 8500-09-0005
Principal Display Panel – 50 mL Caramel Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Caramel/5.5
DERDS01 8500-09-0015
Principal Display Panel – 50 mL Caramel Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Caramel/5.5
DERDS01 8500-09-0015
Principal Display Panel – 50 mL Espresso Box Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
50mL/1.69 Fl. Oz.
Espresso/6
DERDS01 8500-09-0006
Principal Display Panel – 50 mL Espresso Label
RODAN+FIELDS
RADIANT DEFENSE
PERFECTING LIQUID
BROAD SPECTRUM SPF 30
Espresso/6
DERDS01 8500-09-0006