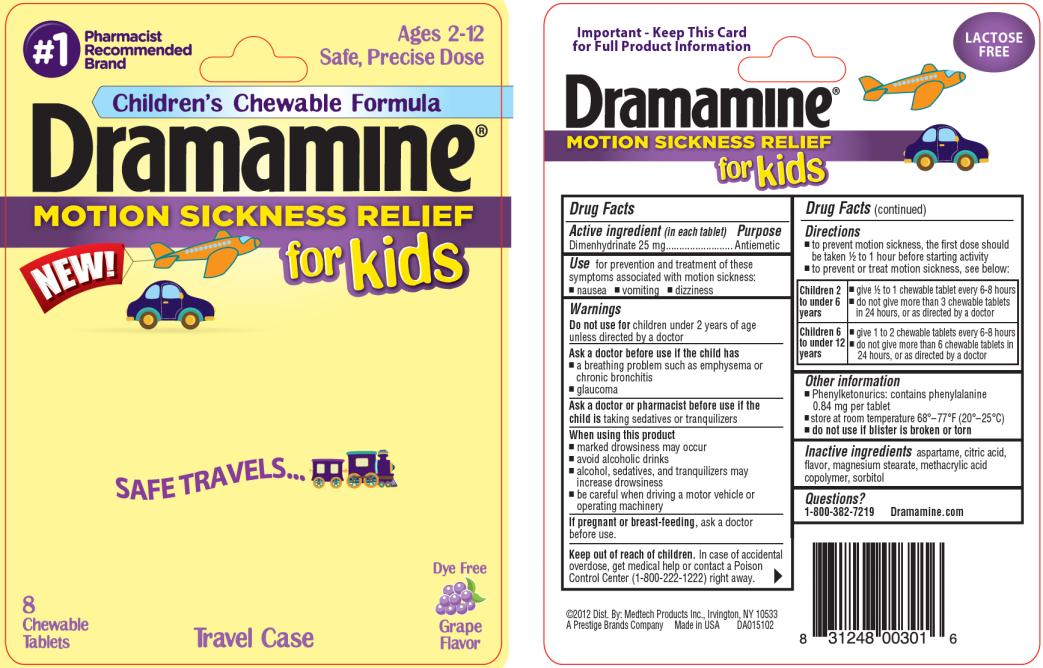
DRAMAMINE FOR KIDS- dimenhydrinate tablet, chewable
Medtech Products Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
(in each tablet)
Dimenhydrinate 25 mg
Use
for prevention and treatment of these symptoms associated with motion sickness:
- nausea
- vomiting
- dizziness
Warnings
Do not use for
children under 2 years of age unless directed by a doctor
Ask a doctor before use if the child has
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use if the child is
taking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding,
ask a doctor before use.
Keep out of reach of children.
In case of accidental overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- to prevent motion sickness, the first dose should be taken ½ to 1 hour before starting activity
- to prevent or treat motion sickness, see below:
Children 2
to under 6
years |
- give ½ to 1 chewable tablet every 6-8 hours
- do not give more than 3 chewable tablets
in 24 hours, or as directed by a doctor
|
Children 6
to under 12
years |
- give 1 to 2 chewable tablets every 6-8 hours
- do not give more than 6 chewable tablets in
24 hours, or as directed by a doctor
|
Other information
- Phenylketonurics: contains phenylalanine 0.84 mg per tablet
- store at room temperature 68º - 77ºF (20º - 25ºC)
-
do not use if blister is broken or torn
Inactive ingredients
aspartame, citric acid, flavor, magnesium stearate, methacrylic acid copolymer, sorbitol
Questions?
1-800-382-7219 Dramamine.com
PRINCIPAL DISPLAY PANEL
#1Pharmacist Recommended Brand
Ages 2 – 12
Safe, Precise Dose
Children’s Chewable Formula
Dramamine MOTION SICKNESS RELIEF for Kids
8 Chewable Tablets
Dye Free Grape Flavor
Medtech Products Inc.
